4 Types of Chemical Reactions
What happened to heat energy in the reaction? ( thermic-of or pertaining to heat)
Endothermic
• Endo
-from the Greek meaning inner
Energy is absorbed and held in during this type of reaction.
Exothermic
• Exo
-from the Greek meaning outer
Energy is released and given off during this type of reaction.
Chemistry in a bag experiments
Chemistry in a bag experiments
Types of chemical reactions
4 types
Chemical equation a short easy way to show a chemical reaction, using symbols instead of words
2Cu + O
2
REACTANTS
2H
2
0
2
REACTANTS
--- 2CuO
PRODUCTS
--- 2H
2
O + O
2
PRODUCTS
4 types of chemical reactions
• 1)
Synthesis reaction-
• 2 or more simpler substances combining to produce a more complex substance
2)
Decomposition Reaction
2H
2
0
2
REACTANT
---------- 2H
2
O + O
2
PRODUCTS
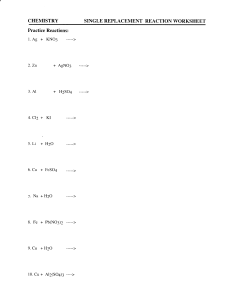
3) Replacement Reaction
When on element replaces another in a compound OR when 2 elements in different compounds trade places
Metals lab ( a single replacement experiment)
Mg + HCl--------- Mg Cl + H
2
The Mg replaced the Hydrogen and formed a new chemical bond with Chlorine.
Hydrogen went back to being a gas. We proved it was H gas by using the wood splint test.
4. Double Replacement