docx
advertisement

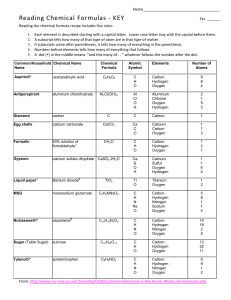
Stoichiometry Unit 7 Worksheet 1 Date__________Name ______________________________ Show all work needed on a separate sheet of paper. Part 1: 1. How many grams of magnesium are required to react with 0.350 mol hydrochloric acid? Mg + 2HCl MgCl2 + H2 2. How many moles of calcium nitrate would be produced from 0.877mol nitric acid? Ca3(PO4)2 + 6HNO3 3Ca(NO3)2 + 2H3PO4 Part 2: 3. Determine the mass of calcium hydroxide produced when calcium carbide reacts with 0.695g of water. CaC2 + 2H2O Ca(OH)2 + C2H2 4. What mass of hydrogen peroxide (H2O2) must decompose to produce 0.241g of water? 2H2O2 2H2O + O2 Part 3: 5. How many mols of ozone must decompose to produce 0.870L of oxygen? 2O3 3O2 6. 2.66L of carbon dioxide will form at STP from what mass of benzene in a combustion reaction? 2C6H6 + 15O2 6H2O + 12CO2 Part 4: 7. What volume of hydrogen sulfide (H2S) is necessary to react with 17.4 mL of oxygen gas? 2H2S + 3O2 2SO2 + 2H2O 8. What volume of nitrogen should be produced with 5.8L of carbon monoxide (CO)? 2C7H5(NO2)3 2C + 12CO + 5H2 + 3N2 Part 5: 9. If 2.63L of oxygen are consumed in the combustion of acetylene (C2H2), how many molecules of carbon dioxide should be produced? 2C2H2 + 5O2 2H2O + 4CO2 10. Find the mass of aluminum required to produce 4.72x1023 formula units of aluminum sulfate. 2Al + 3H2SO4 Al2(SO4)3 + 3H2 Part 6: Nitrogen (N2) (round) and hydrogen (H2) (square) react to form ammonia (NH3). Consider the mixture of nitrogen and hydrogen in the picture below. Draw another picture to represent the product mixture, assuming the reaction goes to completion. How did you arrive at your pictorial representation? N2 + 3H2 2NH3