Types of Chemical Reactions
advertisement

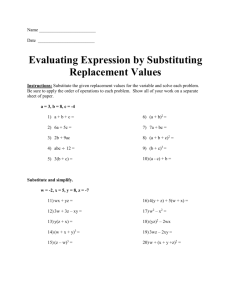
Types of Chemical Reactions A chemical reaction is when one or more substances are changing in to different substances. Typical chemical reactions include burning, decay, fermentation, corrosion of steel and digestion of food. There are six common types of reactions: Synthesis, Decomposition, Single Replacement, Double Replacement, Combustion, and Neutralization. Synthesis A reaction that combines two or more reactants to form one product. General Formula: A+B→AB Eg. 2Fe + 1O₂ → 2FeO Decomposition A reaction that breaks down one reactant into two or more products. General Formula: BC→B+C Eg. 2Ag₂O→4Ag+1O₂ Single Replacement (displacement) A reaction where an ionic element replace positive ions and non-metal elements replace negative ions. General Formula: A+BC→AC+B (A=metal) Eg. 2CuO+1C→2Cu+1CO₂ or A+BC→BA+C (A=non-metal) *Predicting Single Replacement Reactions sometimes, single replacement can not be performed because some metals are more reactive than others and so some metals. to check if the reaction is possible to happen, use the "ACTIVITY SERIES"ーAn element higher up on the series replaces the ion below it on the table. Eg.1Cl2 + 1NaBr 1NaCl + Br2 On the Activity Series, Bromine (Which replaces the Chlorine) is located higher than Chlorine. Therefore, this reaction can be performed. Double Replacement a reaction between two ionic compounds usually in solution. the ions switch partners General Formula: AB+CD→CB+AD Eg.1Na2CO3 +1CaCl2 → 1CaCO3 + 2NaCl *Using "Table of Solubilities" you can check if the reactions of double replacement occurs by determining the states(aq) or (s) from using the "Table of solubilities" If the reactants change state during the reaction, there is a reaction. Combustion a reaction where burning in air is involved. the oxygen atoms usually combine with more than one type of atom as products. General Formula: AB+O₂→AO+BO Eg. 1C3H8 + 5O2 → 3CO2 + 4H2O Neutralization it is one kind of double replacement reaction where acids react with bases to produce water and an ionic salt as product General Formula: HA+BOH→H₂O+BA Eg.1HCl + 1NaOH → 1NaCl + 1H2O