Counting Atoms and Balancing Chemical Equations
advertisement
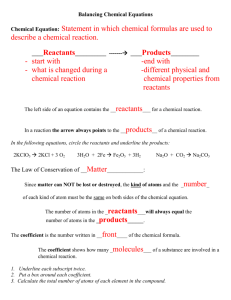
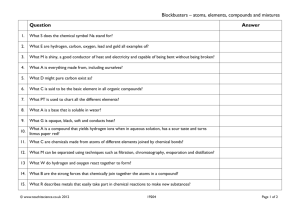
Counting Atoms and Balancing Chemical Equations Write what is in Blue Elements, Compounds, Mixtures Hydrogen is an element. Oxygen is an element. When hydrogen and oxygen bond they make the compound water. When salt and water are combined, a mixture is created. Compounds in mixtures retain their individual properties. The ocean is a mixture. Elements, compounds, and mixtures Mixtures can be separated by physical means. Compounds can only be separated by chemical means. Elements are pure substances. When the subatomic particles of an element are separated from its atom, it no longer retains the properties of that element. Identifying Compounds Each new element is identified by a capital letter Example: H2SO4 The elements in Sulfuric Acid Hydrogen Sulfur Oxygen Subscripts C12H22O11 There are 12 atoms of Carbon There are 22 atoms of Hydrogen There are 11 atoms of Oxygen If there is not a subscript listed, it is understood to be 1. Example: NaCl There is one atom of Sodium There is one atom of Chlorine You Practice! NaHCO3 HCl Sodium – 1 Hydrogen – 1 Carbon – 1 Oxygen -3 Hydrogen – 1 Chlorine - 1 There are times you will see a compound with parenthesis. Pb(NO3)2 The 2 after the parenthesis indicates there are two sets of the parenthesis. Pb(NO3) (NO3) So, in counting the atoms, you would have the following: Lead – 1 Oxygen - 6 Nitrogen -2 You Practice!! (NH4)3PO4 Mg(OH)2 Nitrogen – 3 Hydrogen – 12 Phosphorus – 1 Oxygen – 4 Magnesium – 1 Oxygen – 2 Hydrogen – 2 Coefficient 2H2SO4 This means there are 2 compounds of Sulfuric Acid. Think: H2SO4 H2SO4 Counting the atoms: Hydrogen – 4 Sulfur – 2 Oxygen - 8 You Practice!! 3H3PO4 2H2O Hydrogen – 9 Phosphorus – 3 Oxygen - 12 Hydrogen – 4 Oxygen - 2 Got It???? Chemical Equations Chemical equations express what is happening in a chemical reaction using symbols. 4K + Cl2 Reactant 2KCl Product Law of Conservation of Mass In a chemical reaction, matter cannot be created or destroyed. It can only be changed. What does that mean?? The reactants MUST contain the same elements and the same number of each element as the product. They CAN be in different compounds. HUH????? H2 + O2 Reactants: H = 2 Products: H = 2 H2O O=2 O=1 This cannot happen. An oxygen atom cannot be destroyed. So what do we do? The chemical equation must be written according to the law. AGAIN --- HUH??? H 2 + O2 H2O is the production of water. Well, how it actually happens in nature is this…. 2H2 + O2 2H2O Reactants: H = 4 O = 2 Products: H = 4 O = 2 Do I Need to Know How to Balance? NOT YET. You need to be able to recognize if the chemical reaction follows the law of conservation of mass. Na + O2 Mg + 2HCl Na2O NOPE MgCl2 + H2 YUP!! You Practice!!! HgO N2 + 3H2 Zn + 2HCl Hg + O2 NOPE 2NH3 YUP ZnCl2 + H2 YUP More Practice K + Br2 2KBr 2Fe + O2 6Fe2O3 2Na + 2H2O NOPE NOPE 2NaOH + H2 YUP Got It??? Warm Up 3/9 Is it balanced? Show your work!! Al + O2 ---> Al2O3 SiCl4 + H2O ---> H4SiO4 + HCl Balancing Chemical Equations H2 + O2 Reactants: H = 2 O=2 H2O Products: H = 2 O=1 What do we multiply by 1 to get 2? Keep Going…. H2 + O2 Reactants: 2H2O Products: 2=H = 4 2 =O = 2 Now the Oxygen is balanced but Hydrogen is not, so what do we multiply by 2 to get 4? Almost There!!! 2H2 + O2 2H2O Reactants: Products: 4 H=4 2=O = 2 Now it is balanced!!! Congratulations, you have now learned the hardest thing we will do all year!! Now You Practice HgO Hg + O2 =Hg = =O = K + Br2 2KBr =K = =Br = Warm Up 3/11 Write Q&A Why do we have to balance equations? (What law do we have to follow?) Warm Up 3/10 Write Q&A Balance the Following: HNO3 + Ca(OH)2 Ca(NO3)2 + H2O Warm Up 3/8 Write the Following: The Law of Conservation of Mass In a chemical reaction, matter cannot be created or destroyed. It can only be changed. Warm Up 3/5 Write Q&A How many atoms are in 3Pb(OH)4? Pb O H Warm Up March 4 Write Q&A The vertical columns on the periodic table are called ______________ and they correspond to the number of __________ The horizontal rows on the periodic table are called ____________ and they correspond to the number of __________