Balancing Chemical Equations: Conservation of Mass
advertisement

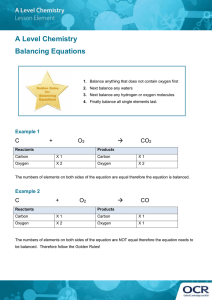
Conserving Mass in a Chemical Reaction and Balancing In a chemical equation, the number of each atom on the reactants side has to equal the number of that same atom on the products because a chemical reaction must obey the Law of Conservation of Mass. This law states: Matter________________________________________________________. Or more simply, the mass of substances produced (products) is always equal to the mass of the reacting substances (reactants). “What goes in must come out", although it may be in a different form (state, compound) the amount of mass of each atom is the same. *(The number of each elements on the reactants side has to equal the same number on the products side)* Ex. C(s) + O2(g) -----> CO2(g) C: O: Is this equation balanced from Reactants to Products side? C: O: In order to balance a chemical equation, we must place ____________ (big numbers out in front of chemical formulas) . You cannot change subscripts or add numbers anywhere in a formula except for the front. DO NOT CHANGE formulas! Coefficients apply to ______________ in a chemical formula. Rules for Balancing Chemical Equations 1. Write out the skeleton equation (unbalanced) and make sure that you have written the formula for each molecule correctly. 2. Balance atoms that occur in the largest number first. Leave oxygen and hydrogen until the end. 3. Balance polyatomic ions next (only if they are the same on both sides) ex: SO42- must be equal on both sides. 4. Balance hydrogens and oxygens last. 5. Write the state of each molecule or element. 6. Check your answer! All coefficients should be in lowest terms. ex. H2 ex. Mg + + O2 ----> O2 ----> H2O MgO ex. CH4 + ex. O2 Zn + -----> H2O AgNO3 ----> Zn(NO3)2 + Ag More Balancing KI + Pb(NO3)2 ----> CaSO4 HCl + AlBr3 + C3H8 + Ba3(PO4)2 Ca(OH)2 O2 -----> + KNO3 ------> ------> CO2 KBr ---> + + PbI2 Al2(SO4)3 H2O + + CaBr2 CaCl2 H2O K3PO4) + BaBr2