AP Biology ch. 5
advertisement
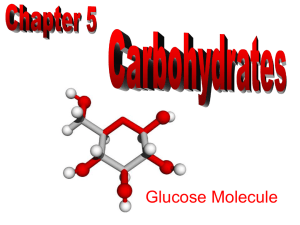
AP Biology: Chapter 5 Structure and Function of Macromolecules: CARBOHYDRATES Polymer Principles • Most macromolecules are polymers • Polymer-large molecule consisting of many identical or similar subunits connected together. • Monomer- subunit or building block of a polymer • Macromolecule- large organic polymer Figure 5.1 Building models to study the structure and function of macromolecules Classes of Macromolecule in living organisms • Carbohydrates- used as fuel and building material • Lipids-energy storage • Proteins-structure, movement, enzymes • Nucleic acids-store and transmit hereditary information. Polymerization Reactions • Formation of macromolecules from smaller building block molecules represents another level in the hierarchy of biological organization. • Most polymerization reactions are condensation (dehydration) reactions. Condensation Reactions • Monomers are covalently linked with the removal of a water molecule at each linkage • One monomer loses a hydroxyl (-OH), and the other monomer loses a hydrogen (-H). • Process requires energy, and the presence of a biological catalyst Hydrolysis Reaction • Reaction that breaks covalent bonds between monomers by the addition of water molecules. • A H+ from the water bonds to one monomer, and the OH- bonds to the adjacent monomer. • Example: Hydrolytic reactions break down food molecules into monomers that can be absorbed in the blood. Carbohydrates • Sugars and starches used for fuel and building material • Monosaccharides- simple sugar building blocks (monomers) • Polymers are chains of monomers formed by condensation reactions • Carbohydrates are classified by the number of simple sugars in the compound Monosaccharides • Simple sugar in which C, H, and O are in a ration of 1:2:1 (CH2O) • Can be produced by photosynthetic organisms from CO2, H2O, and sunlight (glucose) • Cellular respiration releases energy in chemical bonds. • Monomers for disaccharides and polysaccharides. Characteristics of Sugars • --OH group attached to each carbon except one, which has a carbonyl group (C=O) • Size of the carbon skeleton varies from 3-7 carbons • Spatial arrangement may vary, such as with enantiomers • In aqueous solution, most monosaccharides form rings. Disaccharides • Double sugar formed from two monosaccharides joined by a glycosidic linkage. • Examples: Maltose = glucose + glucose (beer) Lactose = glucose + galactose (milk) Sucrose = glucose + fructose (table sugar) Polysaccharides • Polymers of sugars, have storage and structural roles • Formed by linking monomers by condensation reactions • Important biological functions: energy storage (starch and glycogen) and structural support (cellulose and chitin) Storage polysaccharides • Starch and glycogen can be hydrolyzed into sugars as needed. • Starch is stored in plants in plastids. Most animals have enzymes to digest starch. • Glycogen is stored in the muscles and liver of animals. Structural polysaccharides • Cellulose: major component of plant cell walls, cannot be digested by most organisms because of B 1-4 linkage • Chitin: polymer of amino sugar, forms exoskeletons of arthropods, cell walls of fungi