carbon notes
advertisement

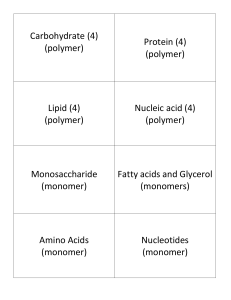
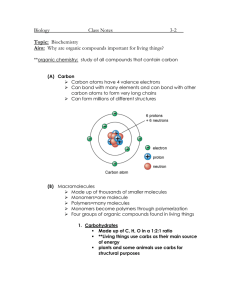
ORGANIC COMPOUNDS SECTION 1-6 P.33-34 CHARACTERISTICS OF ORGANIC COMPOUNDS 1. THEY ARE CARBON-BASED COMPOUNDS (SOME, SUCH AS CARBON DIOXIDE ARE NOT INCLUDED AS NO HYDROGEN IS PRESENT) 2. CARBON HAS 4 VALENCE ELECTRONS WHICH FORM COVALENT BONDS (TO CARBON AND OTHER ELEMENTS) 3. THEY MAKE UP HYDROCARBONS (WHICH CONTAIN ONLY HYDROGEN AND CARBON) SUCH AS METHANEFUEL 4. THEY ARE BASED UPON SINGLE UNITS CALLED MONOMERSMONOSACCHARIDES (CARBS) 5. THEY FORM CHAINS OF MONOMERS CALLED POLYMERS (ALSO CALLED MACROMOLECULES) CARBOHYDRATES TYPES OF CHEMICAL REACTIONS 1. DEHYDRATION SYNTHESIS *COMBINING MONOMERS TO FORM A POLYMER AND PRODUCING WATER MONOMER + MONOMER POLYMER + WATER 2. HYDROLYSIS *USING WATER TO BREAK APART POLYMERS INTO MONOMERS POLYMER + WATER MONOMER + MONOMER *SEE HANDOUT FOR EXAMPLES*