Exam 3 practice B
advertisement

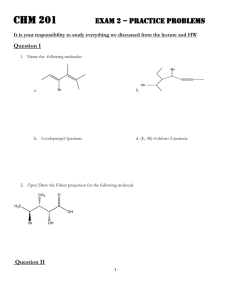
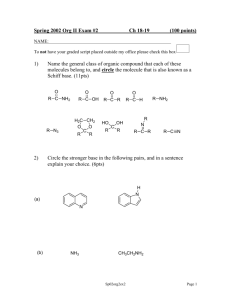
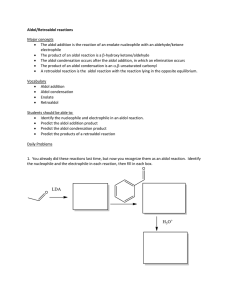
Name_____________________________________________________ Exam 3 R340 Fall 2012 1. (6pts) Propose a mechanism for this nucleophilic addition. 2. (6pts) The following aldol reaction was conducted and the aldol addition product was formed. After further heating, the aldol condensation product formed. Draw structures for both of these products. 3. (12pts) Draw the products of these condensation reactions and label them either “aldol condensation”, “imine formation” or “acetal formation.” 4. (10pts) A. Indicate the acetal functional group in this disaccharide. B. Indicate the hemiacetal functional group in this disaccharide. C. Label the dissacharide linkage, using alpha or beta and the numbers of the carbon atoms that are linked. D. Draw the two monosaccharides of the hydrolysis reaction in the boxes 5. (10pts) Draw a mechanism for this reaction. Draw the energy diagram. Is the reaction endothermic or exothermic? 6. (16 pts) Choose 4 of the following 5 reactions. Clearly mark the one you do not want graded or else the first four will be graded. Given the mechanism, predict the product. 7. (6pts) Predict the product of these hydrolysis reactions based on the different conditions. 8. (12pts) Label each reaction as a nucleophilic substitution, a nucleophilic addition, or a nucleophilic acyl substitution. Then predict the product. 9. (6pts) Circle every compound that is susceptible to hydrolysis. 10. (10pts) Label the phosphate anhydrides, phosphate monoesters, and phosphate diesters in these molecules. Indicate all bonds that are considered “high energy” bonds. 11. (6pts) The full structure of acetyl Coenzyme A is shown below. A. Label one bond as “most susceptible to hydrolysis.” B. Label the two phosphate functional groups as either “kinetically stable” or “kinetically unstable.” NH2 O N N O S OO O NH O OH N P P O O OO H N H H O H H O O H P O- O- N