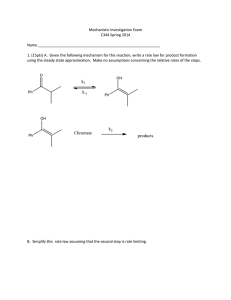
Sp02
advertisement

Spring 2002 Org II Exam #2 Ch 18-19 (100 points) NAME: To not have your graded script placed outside my office please check this box 1) Name the general class of organic compound that each of these molecules belong to, and circle the molecule that is also known as a Schiff base. (11pts) O R C NH2 R N3 2) O R C OH H2C CH2 O O C R R O R C R HO R C OH R O R C H R N R C R R NH2 R C N Circle the stronger base in the following pairs, and in a sentence explain your choice. (6pts) H N (a) N (b) NH3 CH3CH2NH2 Sp02org2ex2 Page 1 3) Draw the Lewis structure (including lone pairs) for the following molecules. (12pts) R NO2 R CN R NCO PPh3 4) Name the following compounds in IUPAC acceptable terms. (15pts) H3C O C H H3C HN C O O Sp02org2ex2 Page 2 5) Aniline (Ph-NH2) reacts with nitrous acid (HONO) to form a diazonium salt. Draw a Lewis structure (with lone pairs) for nitrous acid. (2pts) During the reaction, Nitrous acid undergoes acid catalyzed dehydration to produce the nitrosonium cation (NO+). Draw the mechanism for this transformation, and show that the nitrosonium cation is resonance stabilized. (8pts) HONO H+ H2O N O+ Sp02org2ex2 Page 3 6) What is the definition of a condensation reaction? (2pts) Provide a chemical reaction that is an example of a condensation reaction. (2pts) What is the definition of a protecting group? (2pts) Provide an example of a protecting group and the group it protects. (3pts) Draw a synthetic scheme that requires and exemplifies the use of a protecting group. (6pts) Sp02org2ex2 Page 4 7) Give the products formed in five of the following reactions. (15pts) O 1)Ph3P, CH3-Br (a) 2) BuLi 3) warm O (b) (c) (d) 1) PhMgBr H C H 2) H3O+ O H C H Ph NH2 Ph NH2 H+ 1) NaNO2, HCl 2) CuBr, HBr, heat NH (e) Ph 1) excess CH3-Br 2) Ag2O, H2O, heat (f) H3C NH2 O excess F3C C Cl Sp02org2ex2 Page 5 8) Explain why aldehydes and ketones undergo nucleophilic addition, but acid chlorides undergo nucleophilic acyl substitution. (9pts) Nucleophilic Addition O R C R CH3OH O H R C OCH3 R O Nucleophilic Acyl Substitution CH3OH R C Cl O R C OCH3 Sp02org2ex2 H+ Cl- Page 6 9) Explain why a ketone must have three or more carbon atoms. (7pts) *Bonus question* (up to 4pts) For the reaction of a primary amine with a ketone, the optimum pH for imine formation is around 4.5. Explain why this reaction goes more slowly both at lower and higher pH. Sp02org2ex2 Page 7