CHM 201-EXAM2-practice problems
advertisement
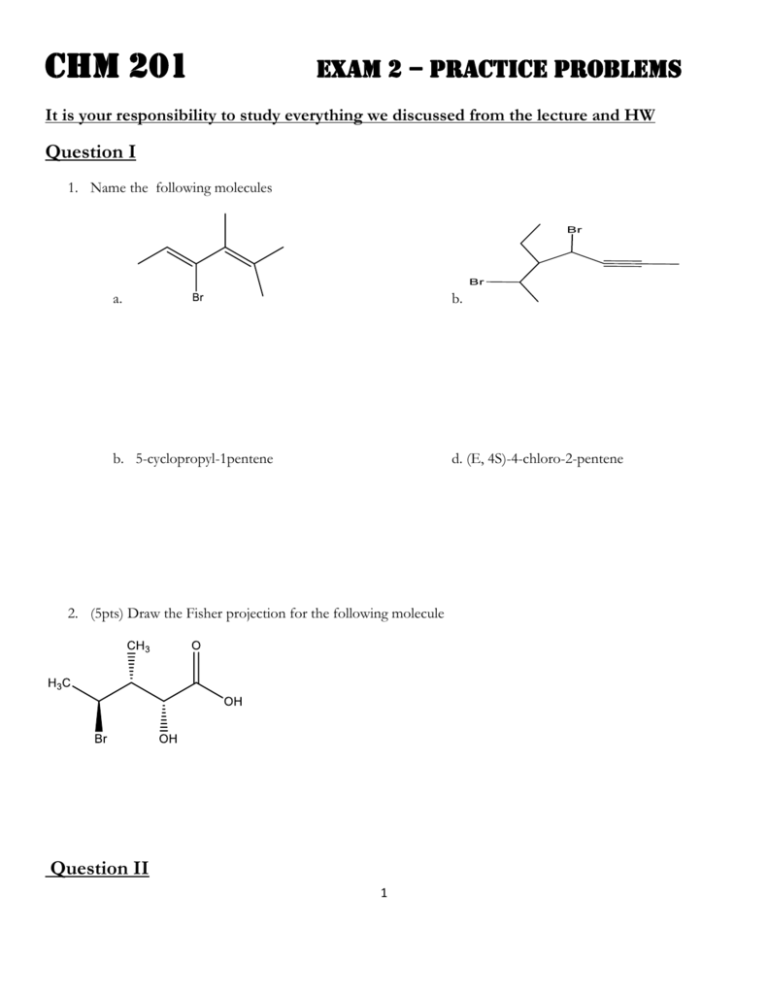
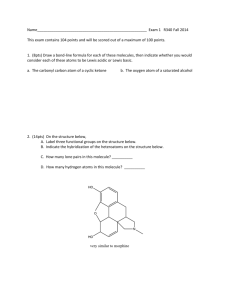
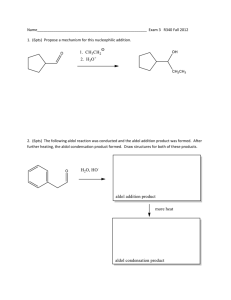
CHM 201 EXAM 2 – practice Problems It is your responsibility to study everything we discussed from the lecture and HW Question I 1. Name the following molecules a. b. b. 5-cyclopropyl-1pentene d. (E, 4S)-4-chloro-2-pentene 2. (5pts) Draw the Fisher projection for the following molecule Question II 1 1. (12pts) Assign R, S, E, Z configuration for the following molecule. Show your work to receive full credit – i.e… show the priority of each group a. b. c. 2. 3. Draw all Fisher projections for 2,3-dichloropentane then answer the following questions a. pairs of enantiomeric structures b. pair of diastereomeric structures c. pairs of identical structures d. total numbers of stereisomers possible for this molecule Question III 2 1. (6pts) 2. (12pts) Looking down from C2-C3 and starting at the lowest energy conformer, draw all conformers for 2bromobutane. Then draw an energy versus dihedral angle plot to show their energies differences. Assume bromide group is energetically less expensive than those alkyl groups. Question IV 1. (12tps) Predict the major product(s). If no reaction occurs then write NR 3 b. c. d. e. 2. (6pts) Starting with an alkyne(s) and all possible reagents, indicate how the following compound can be prepared. Draw them out. Question V 4 (13pts) The hydration of 2-methylpentene gives two products as shown in the following reaction. Which one of the products formed is the major product? Explain your choices. Write the complete mechanism to support your choice by providing any intermediates that may exist. Fill in missing electrons, formal charges and show correct arrow pushing for full credit 5