Robinson Annulation Experiment: CHM556 Instruction Sheet
advertisement

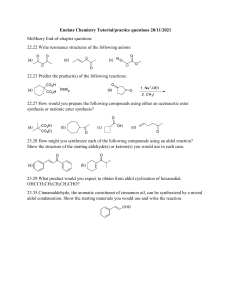
Instruction sheet for students. CHM556 Experiment 5 The Robinson Annulation Reaction For background of this experiment, refer to experiment 39, page 680 of the laboratory textbook: Introduction to Organic Laboratory Techniques, A Small Scale Approach, 3 rd Edition by Engel, Kriz, Lapman and Pavia. In this experiment, initially, you will perform a sodium-catalyzed conjugate addition of ethyl acetoacetate to previously prepared trans-chalcone from experiment 4 via a Michael addition reaction. Then, the reaction will proceed with a based-catalyzed aldol condensation reaction and finally, the aldol intermediate will be dehydrated to form an ,-unsaturated ketone as the final product. Conduct the experiment using the experimental procedures described in the text. You are required to utilize the trans-chalcone that you have prepared in your last experiment (Experiment 4). At the end of the laboratory session, you need to: a) Weigh the dry pure product and determine the percent yield. b) Measure the melting point and obtain a proton NMR spectrum of the product. Your report for this experiment should include: a) The chemical equation of this reaction. Also include the mechanism in your discussion. b) Percentage yields of the crude and purified products. c) Interpretation of the NMR spectrum of your pure product. Comments on the success of the reaction. Organic Chemistry Section, Center of Chemistry and Environmental Studies, Faculty of Applied Sciences, Universiti Teknologi MARA.