Electron Configurations Worksheet - Advanced Chemistry
advertisement
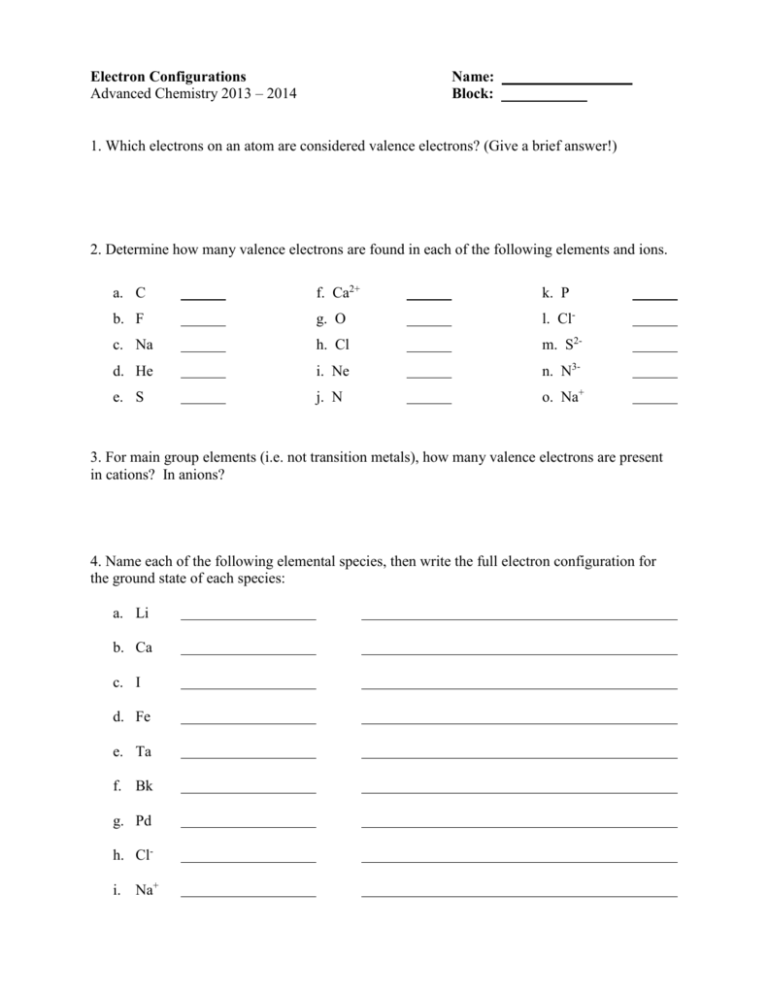
Electron Configurations Advanced Chemistry 2013 – 2014 Name: Block: 1. Which electrons on an atom are considered valence electrons? (Give a brief answer!) 2. Determine how many valence electrons are found in each of the following elements and ions. a. C f. Ca2+ k. P b. F g. O l. Cl- c. Na h. Cl m. S2- d. He i. Ne n. N3- e. S j. N o. Na+ 3. For main group elements (i.e. not transition metals), how many valence electrons are present in cations? In anions? 4. Name each of the following elemental species, then write the full electron configuration for the ground state of each species: a. Li b. Ca c. I d. Fe e. Ta f. Bk g. Pd h. Cli. Na+ 5. Name each of the following elemental species, then write the shorthand (abbreviated) electron configuration for the ground state of each species: a. Ar b. K c. Sr d. C e. If. V5+ 6. Match each electron configuration or atomic number (Z) in Column 1 with a description in Column 2. All of the elements in Column 1 are neutral. Column 1 Column 2 1s22s22p2 has 3 electrons 1s22s1 has 4 principal energy levels 1s22s22p63s23d104s2 atomic number = 6 Z = 18 has 3 unpaired electrons 1s22s22p63s23p1 is a noble gas Z=7 has 3 valence electrons 7. Determine the element for each of the following orbital populations. Write the atomic symbol.