valence electrons
advertisement
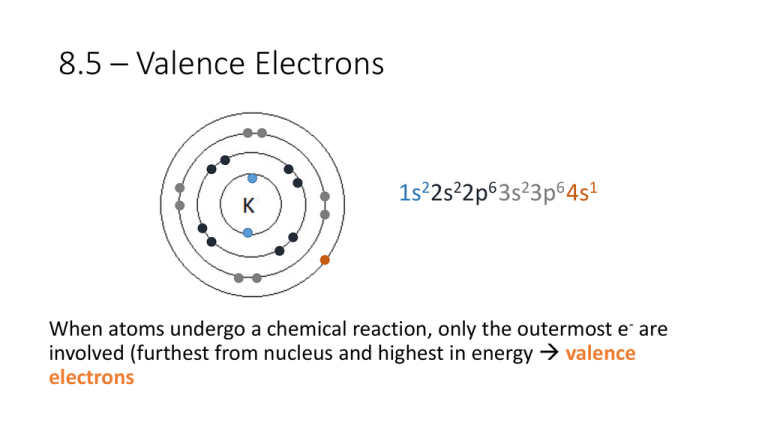
8.5 – Valence Electrons 1s22s22p6 3s23p6 4s1 When atoms undergo a chemical reaction, only the outermost e- are involved (furthest from nucleus and highest in energy valence electrons • Valence electrons are the ones in the s and p subshells beyond the noble gas core, and partially filled d and f subshells • If all subshells are completely full in a atom (i.e. noble gas) then there are no valence electrons Atom Electron Configuration # Valence e- Al [Ne] 3s23p1 3 Ga [Ar] 4s23d104p1 3 Pb [Xe] 6s24f145d106p2 4 Xe [Kr] 5s24d105p6 0 or 8 Can you see a pattern for counting valence electrons on the periodic table? 12 varied 3 4 5 6 7 0 or 8 8.6 – Electron Configuration of Ions O: 2O : 2 2 4 1s 2s 2p ?? For negative ions, add the extra electron(s) where you left off in the neutral atom Example: Write the electron configuration for the following negative ions: a) O2- 1s22s22p4 + 2e- 1s22s22p6 b) S2- 1s22s22p63s23p4 + 2e- 1s22s22p63s23p6 c) Br- [Ar]4s23d104p5 + 1e- [Ar]4s23d104p6 For positive ions, • Electrons in subshells with the _______________ highest n value and highest energy are moved first • Electrons are removed from the ____ s p -subshell first, then ____, and then _____-subshells d 22s22p63s23p64s23d104p65s24d105p2 1s Sn: Sn2+: ?? For positive ions, • Electrons in subshells with the _______________ highest n value and highest energy are moved first • Electrons are removed from the ____ s p -subshell first, then ____, and then _____-subshells d a) Sn2+ [Kr]5s24d105p2 - 2e- [Kr]5s24d10 b) Sn4+ [Kr]5s24d105p2 - 4e- [Kr]4d10 c) V2+ [Ar]4s23d3 - 2e- [Ar]3d3 Example: Write the electron configuration for the following ions: a) O2b) S2c) Brd) Sn2+ e) Sn4+ f) V2+ Neutral Atom 1s22s22p4 1s22s22p63s23p4 [Ar]4s23d104p5 [Kr]5s24d105p2 [Kr]5s24d105p2 [Ar]4s23d3 + 2e+ 2e+ e- 2e- 4e- 2e- → → → → → → Ion 1s22s22p6 1s22s22p63s23p6 [Ar]4s23d104p6 [Kr]5s24d10 [Kr]4d10 [Ar]3d3 Ne Ar Kr When an atom becomes an ion, its electron configuration sometimes becomes the same as the configuration for the nearest ___________________. When two atoms have the same electron noble gas configuration, they are said to be _____________________. isoelectronic Practice: Which of the following is isoelectronic with krypton when ionized? Cl Zn Br Ca Sr Se I Consider this: If two atoms have the same electronic configuration, are they the same element? NO!!! It is the #p that determines the element.





