Chapter 18 Review Phosphorus-32 decays by beta emission to form
advertisement
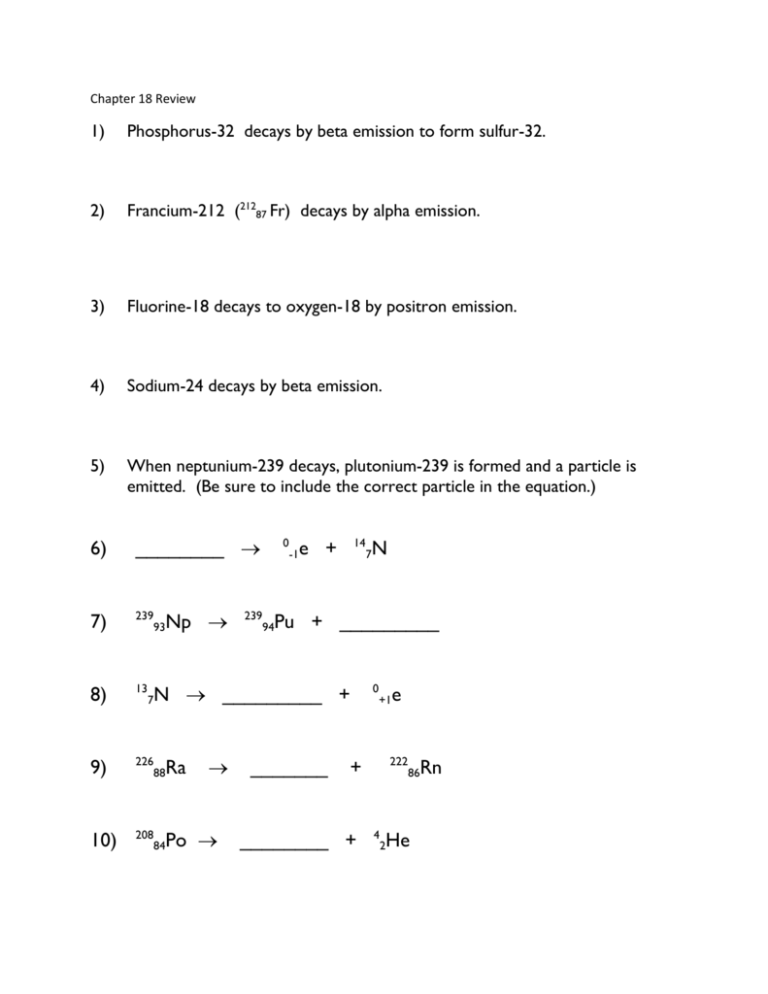
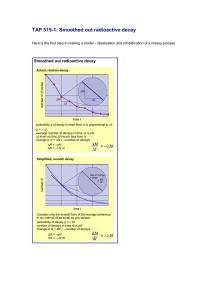
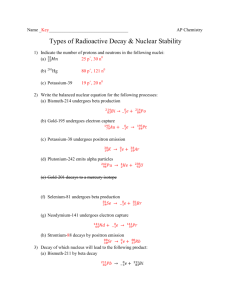
Chapter 18 Review 1) Phosphorus-32 decays by beta emission to form sulfur-32. 2) Francium-212 (21287 Fr) decays by alpha emission. 3) Fluorine-18 decays to oxygen-18 by positron emission. 4) Sodium-24 decays by beta emission. 5) When neptunium-239 decays, plutonium-239 is formed and a particle is emitted. (Be sure to include the correct particle in the equation.) 6) ________ 7) 239 93 8) 13 9) 226 88 Ra 10) 208 84 Po 7 Np 239 0 -1 14 e + N Pu + _________ 94 N _________ + 7 _______ 0 +1 222 + ________ + e 4 2 86 He Rn 11. The half life of a certain isotope is 374 minutes. How long will it take for a 75.0g sample to decay to 2.34g? 12. The first order rate constant for the decay of an isotope is 0.617s-1. What is the half life of the isotope? 13. What type of decay would you expect manganese-45 to undergo? Arsenic-80? Name these compounds Write the formulas