Nuclear equations Background Info
advertisement
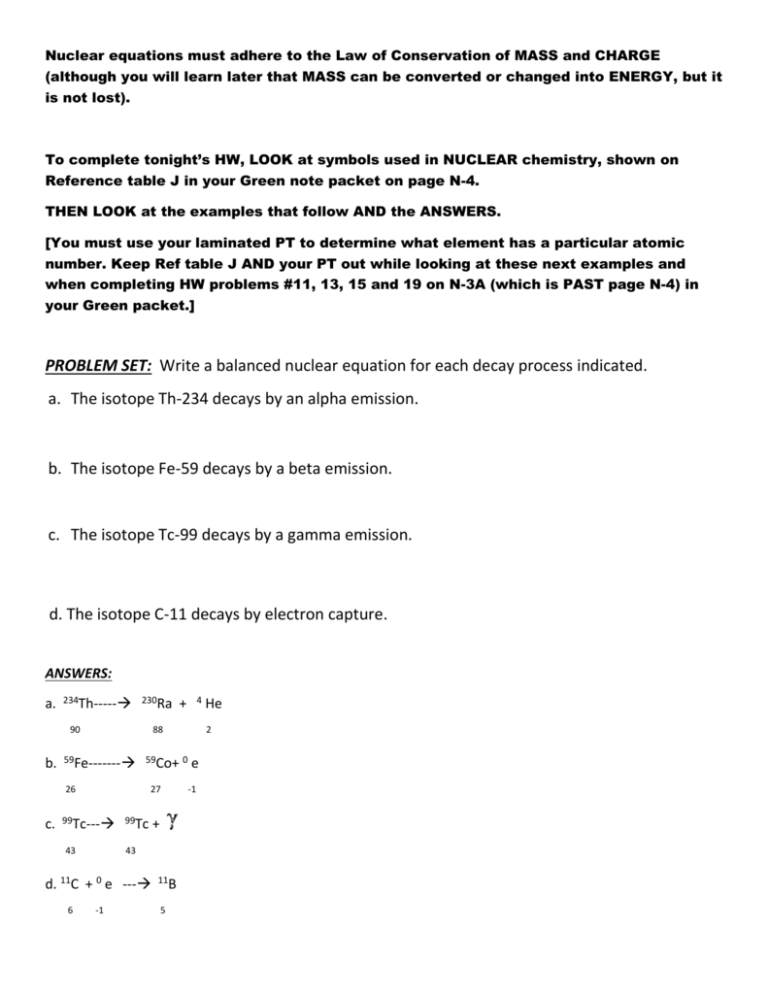
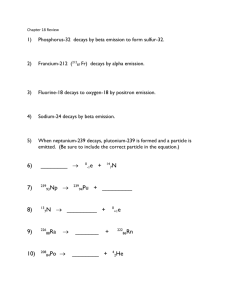
Nuclear equations must adhere to the Law of Conservation of MASS and CHARGE (although you will learn later that MASS can be converted or changed into ENERGY, but it is not lost). To complete tonight’s HW, LOOK at symbols used in NUCLEAR chemistry, shown on Reference table J in your Green note packet on page N-4. THEN LOOK at the examples that follow AND the ANSWERS. [You must use your laminated PT to determine what element has a particular atomic number. Keep Ref table J AND your PT out while looking at these next examples and when completing HW problems #11, 13, 15 and 19 on N-3A (which is PAST page N-4) in your Green packet.] PROBLEM SET: Write a balanced nuclear equation for each decay process indicated. a. The isotope Th-234 decays by an alpha emission. b. The isotope Fe-59 decays by a beta emission. c. The isotope Tc-99 decays by a gamma emission. d. The isotope C-11 decays by electron capture. ANSWERS: a. 234Th----- 230Ra 90 b. 88 59Fe------- 59Co+ 0 26 c. 27 99Tc--- 43 99Tc + 43 d. 11C + 0 e --- 6 4 + -1 11B 5 He 2 e -1