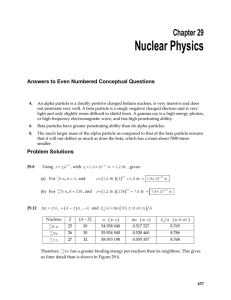
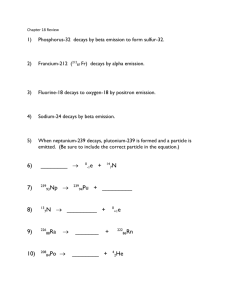
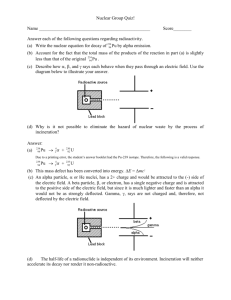
Honors Chem II
advertisement
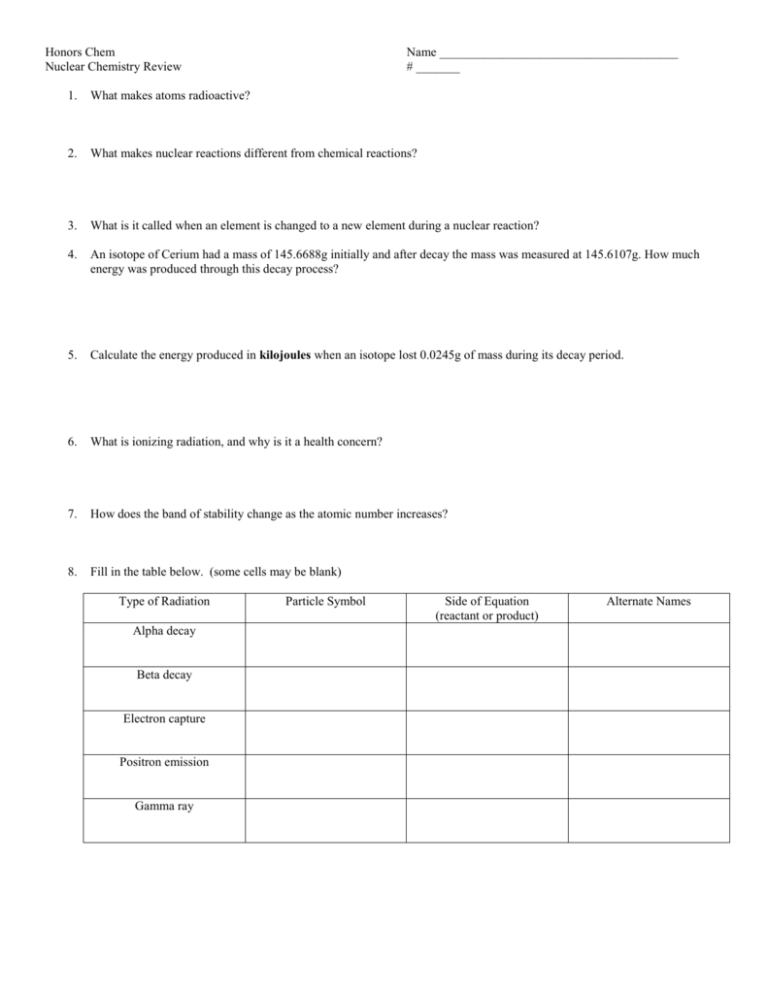
Honors Chem Nuclear Chemistry Review Name ______________________________________ # _______ 1. What makes atoms radioactive? 2. What makes nuclear reactions different from chemical reactions? 3. What is it called when an element is changed to a new element during a nuclear reaction? 4. An isotope of Cerium had a mass of 145.6688g initially and after decay the mass was measured at 145.6107g. How much energy was produced through this decay process? 5. Calculate the energy produced in kilojoules when an isotope lost 0.0245g of mass during its decay period. 6. What is ionizing radiation, and why is it a health concern? 7. How does the band of stability change as the atomic number increases? 8. Fill in the table below. (some cells may be blank) Type of Radiation Alpha decay Beta decay Electron capture Positron emission Gamma ray Particle Symbol Side of Equation (reactant or product) Alternate Names 9. Show all work for each step(s) in the following nuclear reactions. a) Polonium – 210 decays by 2 successive alpha particle emissions. b) Copper – 59 decays by electron capture and then by alpha particle emission. c) Lead – 212 decays by beta negative emission, then by alpha particle emission, and then by positron emission. d) The product of two successive alpha particle emissions is Radium – 235. 10. Define the following terms and give examples of each process: a) Nuclear Fission – b) Nuclear Fusion- 11. A sample contains 0.5 g of radioactive material after 10 half-lives. What was the mass of the original amount of radioactive material? 12. 400 g of a radioactive material decayed over 20 h. What fraction of the original sample is left if the half-life for the material is 5 h? 13. Why are nuclides with short half -lifes always used for medical procedures? 14. Why is C – 14 dating not an accurate way to determine the age of an object older than 50,000 yrs. old? 15. An isotope with an original mass of 22.0g was allowed to decay for 7.7 days. What is the amount remaining if the half life is 3.3 days? (This is bonus level work.)