PH300 HW10 answer key 1)
advertisement

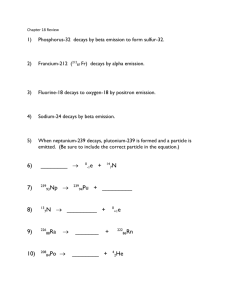
PH300 HW10 answer key 1) T&R ch12 #2 What are the number of neutrons and protons for the following nuclei: 6Li, 15C, 40K, and 64Cu? 6 Li, Z = 3: N = 6 – 3 = 3, 15 C, Z = 6: N = 15 – 6 = 9 40 K, Z = 19: N = 40 – 19 = 21, 64Cu, Z = 29: N = 64 – 29 = 35 2) T&R ch12 #14 Consider two protons in the 27Al nucleus located 2.0 fm apart. How strong does the nuclear force need to be to overcome the Coulomb force? The Coulomb force is described as Fc = kq1q2/r2 Since both charged particles are protons q1 = q2 = 1.602x10-19 C And k = 8.987x109 Nm2/c2 and r = 2.0x10-15 m Fc = (8.987x109 Nm2/c2)*(1.602x10-19 C)*(1.602x10-19 C)/( 2.0x10-15 m)2 = 57.7 N 3) T&R ch12 #17 What is the energy released when three alpha particles combine to form 12C? B(3α→12C) = [3*M(4He) – M(12C)], where M(4He) = 4.002603 u and M(12C) = 12 u So B = 0.007809 u = 7.274 MeV 4) T&R ch12 # 27 Show that the mean (or average) lifetime of a radioactive sample is τ=1/λ=t1/2/ln(2). dN/dt = -λN → − ∫ ∫ dN = λ dt → dt ln(N) = -λt + constant N(t ) = e − λt + constant = N 0e − λt Mean lifetime is time τ such that N0 is reduced by 1/e: N0/e = N0e-λτ ln(1/e) = -λτ → τ = (-1)/(-λ) = 1/λ it checks out. Half life is time t1/2 such that N0 is reduced by ½: N0 = N0e 2 − t1/2 τ ln(1/2) = -t1/2/τ → τ = (-t1/2)/(-ln 2) = (t1/2)/(ln 2) 5) T&R ch12 #31 Tritium (half-life=12.33 y) is mostly produced for military purposes. If we (the US) had stopped producing tritium in 1960 with a 2000-kg stockpile, how much would be left in the year 2010? N0 = 2000 kg, t0 = 0, tf = 2010 – 1960 = 50 years, t1/2 = 12.33 years N(50 ) = − ln (τ )⋅50 2000e 12.33 N(50) = 120.312 kg This answer makes sense because 50 years is approximately 4 half lives, 2000*(1/2)4 = 125 6) T&R ch12 #35 Show directly using masses that protons do not undergo any of the beta decays. Q = [M(parent) – M(daughter)]*c2 β-: Must have neutron parent β+: p+ → n0 + e+ + ν Q = [1.00728u – 1.00866u – 2*(5.4858)x10-4u]*c2 = -0.002477uc2 Capture: p+ + e+ → n0 + ν Q = [1.00728u + 5.4858u - 1.00866u]*c2 = -0.000825uc2 Q < 0 in both cases, proton is stable to beta decay. 7) T&R ch 13#31 Calculate the energy released in kilowatt hours from the fission of 1kg of 235U. Compare this with the energy released from the combustion of 1kg of coal. The heat of combustion of coal is about 29,000 BTU/kg. Assuming 180 MeV/fission (recoverable) 180 ⋅ 10 6 eV 6.022 ⋅ 10 23 atoms 1 mol 1000 g 1.6022 ⋅ 1019 J × × × × = 7.39 ⋅ 1013 J or ~ 1014 J from book 1 atom 1 mol 235 g 1 kg 1 eV 2.053x107 kW*hr What about coal? 1 kg coal × 29000 BTU 1.06 ⋅ 10 3 J × = 3.07 ⋅ 10 7 J 1 kg 1 BTU 8. 54 kW*hr The two fuel sources are not comparable. 8) Using the link http://www.new.ans.org/pi/resources/dosechart/ estimate your yearly radiation dose. Do not include medical doses. Let’s say a person lives on a nuclear submarine. ~50 mrem background (Lots of H20) shielding) ~10-20 mrem reactor proximity (Non-Reactor Room Duty) 70 mrem < 33% of the dose received by someone living in a rain forest Note: This is extremely conservative. In fact, if one were to remain aboard a sub for the life of the reactor (10 to 15 years) the received dose would be less than a lifetime of exposure to cosmic & geological background at sea level. Extra Credit Extra Credit T&R ch12 #61 Radon gas in the form of 222Rn is a health hazard because it is a gas that occurs as a result of one of the naturally occurring radioactive decay chains. It tends to collect in basements and can be inhaled by humans. a) Which decay chain produces this isotope of radon? b) Show that 222Rn produces five more disintegrations before a stable isotope is reached. c) Choose one of the paths of the decay chain from the 222Rn to the stable isotope and sum the half-lives. Approximate the number of days it would take for more than half these decays to occur for a given amount of radon. a) Simplified decay chain: 238 U decays to 2 α and 230Th 230 Th decays to an α and 226Ra 226 Ra decays to an α and 222Rn b) Can show stability/instability for β, α decays at each isotope using binding energies (i.e. Q<0 or Q>0). Step 1. 222Rn decays to an α and 218Po Step 2. 218Po decays to an α and 214Pb Step 3. 214Pb decays via beta emission to 214Bi Step 4. 214Bi decays to an α and 210Tl Step 5. 210Tl decays via beta emission to 210Pb 210 Pb has a half life of 22 years c) Step 1 takes 3.8 days, Step 2 takes 3.1 minutes, Step 3 takes 27 minutes, Step 4 takes 20 minutes and Step 5 takes 1.3 minutes So the total half life is about 4 days, w/ longest half life at Rn, thus Rn’s half-life controls the “feed” to shorter lived isotopes and therefore the production rate of 210Pb.