balancing nuclear eq
advertisement

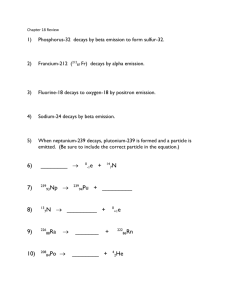
Chemist’s Daily Chapter 22-1 1. Write balanced nuclear equations for the following: 11 6 214 83 237 93 C Bi decays by positron emission: 11 6 C decays by emission of a beta particle 0 → 1 214 83 Np 2. 195 79 39 19 237 produces an alpha particle 93 Np Bi → e → 4 2 11 + 0 -1 5 e He Supply the missing particle: Au K 0 + -1 → 38 19 e K → + 195 78 1 0 Pt neutron Electron capture Neutron emission B 214 + + 233 91 84 Pa Po Chemist’s Daily Chapter 22-2 1. Technetium-99m is used to form pictures of internal organs and is often used to assess heart damage. emission. The "m" indicates an excited nuclear state that decays by gamma The rate constant for decay of technetium-99m is known to be 1.16 x 10 -1 What is the half-life? t1/2 = ln 2 / k = ln 2 / 1.16 x 10 2. -1 h -1 = 5.96 hrs The half-life of molybdenum-99 is 67.0 hours. molybdenum-99 is left after 335 hours? t1/2 = ln 2 / k k = ln 2 / t1/2 = ln 2 / 67 hrs = 0.0103 h ln (No / N) = kt -1 h . How much of a 1.00 mg sample of -1 = (0.0103 h ) (335 h) = -1 ln (No / N) = 3.47 3.47 (No / N) = 32.0 N = No / 32.0 = 1.00 mg / 32.0 = 0.0312 mg 3. The remnants of an ancient fire in a cave in Africa showed a carbon-14 decay rate of 3.1 counts per minute per gram of carbon. Assuming that the decay rate of C-14 in freshly cut wood is 13.6 counts per minute per gram of carbon, calculate the age of the remnants. The half-life of C-14 is 5715 years. Initial activity = Ao = 13.6 counts per min per gram Current activity = A = 3.1 counts per min per gram t1/2 = ln 2 / k = ln 2 / 5715 years = 1.21 x 10 ln (Ao / A) = kt = -4 ln (13.6 / 3.1) = (1.21 x 10 t = 12, 200 years old yr -1 -4 yr ) t -1