Binary Molecular Compounds
advertisement

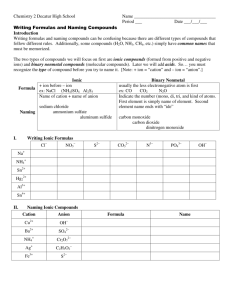
Binary Compounds Containing Two Nonmetals To name these compounds, give the name of the less electronegative element first with the Greek prefix indicating the number of atoms of that element present, followed by the name of the more electronegative nonmetal with the Greek prefix indicating the number of atoms of that element present and with its ending replaced by the suffix –ide. Prefixes you should know: Mono Di Tri Tetra Penta Hexa Hepta Octa Nona Deca 1 2 3 4 5 6 7 8 9 10 Binary Compounds Containing Two Nonmetals (Type III Compounds) As2S3 1. ________________ diarsenic trisulfide SO2 2. ________________ sulfur dioxide P2O5 diphosphorus pentoxide ____________________ CO2 4. ________________ carbon dioxide 3. 5. N2O5 dinitrogen pentoxide ____________________ 6. H2O dihydrogen monoxide ____________________ Prefixes – Binary Molecular Compounds Greek Prefixes for Two Nonmetals Number Indicated 1 2 3 4 5 6 7 8 9 10 Prefixes monoditritetrapentahexaheptaoctanonadeca- Binary Molecular Compounds N2O N2O3 N2O5 dinitrogen monoxide dinitrogen trioxide dinitrogen pentoxide ICl ICl3 iodine monochloride iodine trichloride SO2 SO3 sulfur dioxide sulfur trioxide Naming Binary Compounds Binary Compound? Yes Metal Present? No Type III Use Greek Prefixes Yes Does the metal form more than one cation? No Type I Use the element name for the cation. Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 98 Yes Type II Determine the charge of the cation; use a Roman numeral after the cation name.