molecular-compounds
advertisement

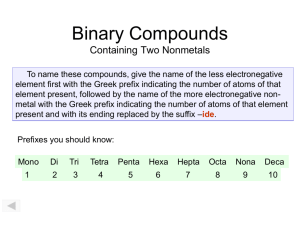
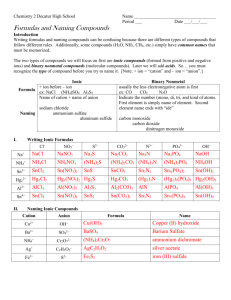
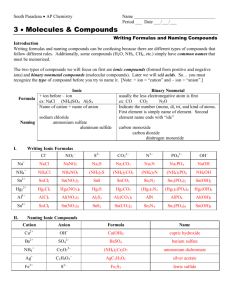
Molecular Compounds SNC2D Molecular Compounds Sometimes non-metals don’t borrow electrons from metals to fill their last shell; instead, they get together and ? Molecular Compounds Sometimes non-metals don’t borrow electrons from metals to fill their last shell; instead, they get together and share their electrons. Molecular Compounds Since they are sharing valence electrons, the bond formed between the atoms is called ? Molecular Compounds Since they are sharing valence electrons, the bond formed between the atoms is called covalent. Molecular Compounds Since they are sharing valence electrons, the bond formed between the atoms is called covalent. These compounds can be represented using Lewis dot diagrams: e.g. Molecular Compounds Or, more commonly, by Kekulé diagrams, where each electron shared is represented by a line connecting the chemical symbols, e.g.: Molecular Compounds Example: Molecular Compounds Example: Multiple Bonds The sharing of more than one electron is represented using more than one line, e.g.: Multiple Bonds The sharing of more than one electron is represented using more than one line, e.g.: There is a double bond between the carbon atoms. Naming Molecular Compounds The elements in the name are given prefixes corresponding to the subscripts (number of atoms) and the second element is given the suffix “-ide.” e.g. CO2 is carbon dioxide The Prefixes 1 2 3 4 5 6 7 The Prefixes 1 2 3 4 5 6 7 Mono-* The Prefixes 1 2 3 4 5 6 7 Mono-* Di- The Prefixes 1 2 3 4 5 6 7 Mono-* DiTri- The Prefixes 1 2 3 4 5 6 7 Mono-* DiTriTetra- The Prefixes 1 2 3 4 5 6 7 Mono-* DiTriTetraPenta- The Prefixes 1 2 3 4 5 6 7 Mono-* DiTriTetraPentaHexa- The Prefixes 1 2 3 4 5 6 7 Mono-* DiTriTetraPentaHexaHepta- The Prefixes 1 Mono-* 2 Di 3 Tri 4 Tetra 5 Penta 6 Hexa 7 Hepta* The 1st element in the name never need a mono Examples OF4 N2O Cl2O7 iodine trichloride diphosphorus pentoxide sulphur hexaiodide Examples OF4 N2O Cl2O7 oxygen tetrafluoride iodine trichloride diphosphorus pentoxide sulphur hexaiodide Examples OF4 N2O Cl2O7 oxygen tetrafluoride dinitrogen monoxide iodine trichloride diphosphorus pentoxide sulphur hexaiodide Examples OF4 N2O Cl2O7 oxygen tetrafluoride dinitrogen monoxide dichlorine heptoxide iodine trichloride diphosphorus pentoxide sulphur hexaiodide Examples OF4 N2O Cl2O7 oxygen tetrafluoride dinitrogen monoxide dichlorine heptoxide iodine trichloride ICl3 diphosphorus pentoxide sulphur hexaiodide Examples OF4 N2O Cl2O7 oxygen tetrafluoride dinitrogen monoxide dichlorine heptoxide iodine trichloride ICl3 diphosphorus pentoxide P2O5 sulphur hexaiodide Examples OF4 N2O Cl2O7 oxygen tetrafluoride dinitrogen monoxide dichlorine heptoxide iodine trichloride ICl3 diphosphorus pentoxide P2O5 sulphur hexaiodide SI6 Non-Conventional Names Some compounds are more commonly known by other names. e.g. NH3 CH4 H2O Non-Conventional Names Some compounds are more commonly known by other names. e.g. NH3 CH4 H2O ammonia Non-Conventional Names Some compounds are more commonly known by other names. e.g. NH3 CH4 H2O ammonia methane Non-Conventional Names Some compounds are more commonly known by other names. e.g. NH3 CH4 H2O ammonia methane water The Diatomic Gases The elemental compounds like H2, Cl2, and O2. are called diatomic gases and are called simply: name of element + “gas” e.g. H2 Cl2 O2 The Diatomic Gases The elemental compounds like H2, Cl2, and O2. are called diatomic gases and are called simply: name of element + “gas” e.g. H2 Cl2 O2 hydrogen gas The Diatomic Gases The elemental compounds like H2, Cl2, and O2. are called diatomic gases and are called simply: name of element + “gas” e.g. H2 Cl2 O2 hydrogen gas chlorine gas The Diatomic Gases The elemental compounds like H2, Cl2, and O2. are called diatomic gases and are called simply: name of element + “gas” e.g. H2 Cl2 O2 hydrogen gas chlorine gas oxygen gas Activity “Modelling Molecules” You need a handout and a modelling kit.