Naming Compounds Sections (Zumdahl 6th Edition) 2.8-2.9 Outline: The Foundation of Stoichiometry
advertisement

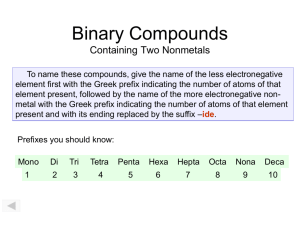
Naming Compounds Sections (Zumdahl 6th Edition) 2.8-2.9 Outline: The Foundation of Stoichiometry The Periodic Table helps organize types Binary Compounds: Metal and Non-Metal Binary Compounds: Two Non-Metals Acids (with and without oxygen) Problems: 2.30-2.35, Naming Exercises 2.36-38, Ions 2.39, 2.42 Discussion Questions (2:1-2.17) You will always have a periodic table available to you when doing problems or taking exams, so start using it now. The Periodic Table • A two dimensional classification scheme for the elements. The columns or groups arrange the elements by chemical classification, as discussed above. The chemical variation across the rows or periods reflect the cyclic variation exhibited in the graphs above. • The periodic table is not only an organizing principle, but it has predictive power as well. • As valuable as the periodic table is, the explanation of its organization was not obvious. It was not until the advent of quantum mechanics that the electronic structure of the atoms could be used to explain the periodic variation in properties. Classification of the Elements • Most of the elements are metals - metallic luster, ability to conduct electricity and heat, and malleability. • The remaining elements are classified as nonmetals - no luster, poor conductors of electricity and heat and brittleness. • There are only 11 non-metals and they are grouped together in the periodic table • Elements in periodic table are laid out by increasing atomic number going across, then down ← 2 ⋅1 → Periodic Table H Li Be NaMg ← K Ca Sc Ti Rb Sr Y Zr Cs Ba La Hf Fr Ra Ac Rf ← ⋅ 3 He → 2 B C N 2 ⋅ 5 → Al Si P V Cr Mn Fe Co Ni Cu Zn Ga Ge As NbMo Tc Ru Rh Pd Ag Cd In Sn Sb Ta W Re Os Ir Pt Au Hg Tl Pb Bi Du Sg Bo HaMe O S Se Te Po F Ne Cl Ar Br Kr I Xe At Rn Ce Pr Nd PmSm Eu Gd Tb Dy Ho Er TmYb Lu Th Pa U Np PuAmCmBk Cf Es FmMd No Lr ← 2 ⋅ 7 → The Alkali Metals The Halogens The Alkaline Earth Metals The Noble Gases Groups in the Periodic Table Main Group Elements (Vertical Groups) Group IA - Alkali Metals Group IIA - Alkaline Earth Metals Group IIIA - Boron Family Group IVA - Carbon Family Group VA - Nitrogen Family Group VIA - Oxygen Family (Calcogens) Group VIIA - Halogens Group VIIIA - Noble Gases Other Groups ( Vertical and Horizontal Groups) Group IB - 8B - Transition Metals Period 6 Group - Lanthanides (Rare Earth Elements) Period 7 Group - Actinides Non-Metals • Chalcogens - (oxygen, sulfur, selenium and tellurium) - form 1:1 compounds with alkaline-earths, but 2:1 compounds with alkali-metals. • Halogens - (fluorine, chlorine, bromine and iodine) - highly reactive and form 1:1 compounds with alkali-metals. • Noble gases - (helium, neon, argon, krypton, radon) - virtually inert to chemical reactions. • Nitrogen (Phosphorous), Carbon and Boron. Naming Ions Transition Metal Ions Naming Binary Compounds (Type I; Ionic) • Binary – only two elements (as ions) combined • Can’t combine two metals (e.g. Cu & Zn –Brass) • The metal ion (cation, or positive) is always named first and the anion second. • A monatomic cation takes its name from the name of the element, e.g. Na+ is called sodium in the names of compounds containing this ion. • The non-metal ion , anion,(or negative ion) is named by taking the first part of the element and adding –ide, e.g. Cl- is chloride. The Periodic Table of the Elements H Li Be Na Mg ← K Ca Sc Ti Rb Sr Y Zr Cs Ba La Hf Fr Ra Ac Rf He F Ne Cl Ar Br Kr I Xe At Rn B C N O 2 ⋅ 5 → Al Si P S V CrMn Fe Co Ni Cu Zn Ga Ge As Se NbMo Tc Ru Rh PdAg Cd In Sn Sb Te Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po Du Sg Bo Ha Me The Transition Metals Ce Pr Nd PmSm Eu Gd Tb DyHo Er TmYb Lu Th Pa U Np PuAmCmBk Cf Es FmMd No Lr Lanthanides: The Rare Earth Elements The Actinides How do we know what charge to make each ion? • The periodic table is our best source of information (a great “cheat sheet”) • Metals in column 1 give up one electron, etc. • Non-metals in column 7 take one electron. • Notice the ions have the number of electrons that the nearest inert gas has. – Å This is the key • Once the charge on each atom is chosen the molecular formula can be determined. Problem: Give the Name and Chemical Formulas of the Compounds formed from the following pairs of Elements (Notice: Some are not simple one-to-one, although they are binary) a) sodium and oxygen Na2O sodium oxide CaF2 calcium fluoride b) zinc and chlorine c) calcium and fluorine d) strontium and nitrogen e) hydrogen and iodine f) scandium and sulfur HI hydrogen iodide (Type II; Ionic) Binary Compounds • Applies to cations that can take on alternate charge states • Non-metals do not take on multiple charges when combining with metals. • When given the molecular formula of the Binary Compound Æ Use the principle of charge balance to determine the cation charge. • Include in the cation name a Roman numeral indicating the charge. • An alternative to the Roman numeral is the name change by adding ‘ic’ or ‘ous’ – stannous fluoride, ferric chloride. Small Molecules F, S, O, N, I, Cl, P Containing Molecules Polyatomic ions The Periodic Table of the Elements H Li Be NaMg K Ca Sc Ti Rb Sr Y Zr Cs Ba La Hf Fr Ra Ac Rf B C N Al Si P V CrMn Fe Co Ni Cu Zn Ga Ge As Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Ta W Re Os Ir Pt Au Hg Tl Pb Bi Du Sg Bo Ha Me O S Se Te Po F Cl Br I At Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np PuAmCm Bk Cf Es FmMd No Lr Boron family Nitrogen family Carbon Family Oxygen Family He Ne Ar Kr Xe Rn Type I Naming Type II Metals Determining Names and Formulas of Ionic Compounds of Elements That Form More Than One Ion. Problem: Give the systematic names for the formulas or the formulas for the names of the following compounds. a) iron (III) sulfide - Fe is 3+, and S is 2- therefore the compound is: Fe2S3 b) CoF2 - c) stannic oxide - Stannic is the common name for tin (IV), Sn4+, the oxide ion is O2-, therefore the formula of the compound is: SnO2 d) NiCl3 - Binary Compounds (Type III); Covalent – contain Two Nonmetals • Two Non-metals can make a molecule (unlike two metals) • How many possible non-metal binary molecules are there? • Covalent is the name given to the type of bond holding them together; distinct from ionic. • The first element in the formula is named using the full element name. • The second element is named as if it were an anion. (just as with ionic compounds). • Prefixes are used to denote the numbers of atoms present. • The prefix mono- is never used for naming the first element, e.g. CO is carbon monoxide. • Which element comes first? (The more metal-like one) Naming Covalent Molecules Examples SO2, sulfur dioxide. CO carbon monoxide CO2 carbon dioxide ClF3 chlorine trifluoride Summary of Binary Compounds • Combine Metal with Metal – Not interesting; just alloys; so 90% of all combinations do not result in a new molecule • Combine Metal with NonMetal – Metal donates; NonMetal accepts electrons – Halides give salts; other nonmetals give brittle compounds. • Combine NonMetal and NonMetal – Both NonMetals must share electrons (unevenly) Polyatomic Ions • Need to develop common acids (and bases) from the binary compounds • From there make polyatomic salts from the acids. • Simple acids are HX, where X is a halogen. Looks like a salt but H is a bit unusual. HCl is a well known acid. Hydrochloric acid • More complex Acids come from nonmetal binary compounds. For example SO2 becomes the acid H2SO3 (sulfurous acid). Just add water. • Distinct from SO3 (sulfur trioxide) becomes H2SO4 (sulfuric acid) a well known acid in water. • Take any binary compound of a non metal and oxygen and add water, and you get an acid (or two distinct) acids (an oxyacid) Specific Molecules You need to know • Rather than continue with the full strategy of naming all compounds lets focus on molecules we will need to discuss reactions. • Already done Col 1 and 2 with Cols 6 and 7, extend this to include Col 5. • Now name specific molecules and anions that use nonmetals. Common Molecules and Names • Notice H with non-metals make acids. • HF, HCl, HBr, HI • Ammonia, Methane, Water, HF – CH4, NH3, H2O, HF • Related ions • Hydronium Ion, Hydroxide Ion, Ammonium Ion H3O+ (or H+) OH- NH4+ • Oxides of non-metals are also acids. – Contrast this with the oxides of metals (CaO) become bases in water (Ca(OH)2) • N, P, and S, Cl form oxides. Oxides of Nonmetals Acids and associated anions Name Formula Hydrochloric acid HCl Hypochlorous acid HClO Chlorous acids HClO2 †Chloric acid HClO3 Perchloric acid HClO4 Anion Name Cl− Chloride ion ClO− Hypochlorite ion ClO2− Chlorite ion ClO3− Chlorate ion ClO4− Perchlorate ion Common Oxyacids and Salts Common molecules to name and recognize Acid Molecules What is common that makes the acid? Ethyl Alcohol and Dimethyl Ether Other molecules that use H, O and C, but are not acids. Examples of Names and Formulas of Oxoanions and Their Compounds - I • KNO2 BaSO3 barium sulfite • Mg(NO3)2 magnesium nitrate Na2SO4 • LiClO4 lithium perchlorate Ca(BrO)2 calcium hypobromite • NaClO3 Al(IO2)3 • RbClO2 rubidium chlorite • CsClO aluminum iodite KBrO3 LiIO4 lithium periodate Examples of Names and Formulas of Oxoanions and their Compounds - II • calcium nitrate • strontium sulfate SrSO4 ammonium sulfite lithium nitrite • potassium hypochlorite KClO lithium perbromate • rubidium chlorate calcium iodite • ammonium chlorite NH4ClO2 • sodium perchlorate (NH4)2SO3 LiBrO4 Ca(IO2)2 boron bromate magnesium hypoiodite Mg(IO)2 Determining Names and Formulas of Anions and Acids Problem 6-4: Name the following anions and give the names and a) I - formulas of the acid solutions derived from them: b) BrO3c) SO3 2- d) NO3e) CN - Solution: a) The anion is b) The anion is bromate; and the acid is bromic acid, HBrO3 c) The anion is d) The anion is nitrate; and the acid is nitric acid, HNO3 e) The anion is Salts of Oxyacids: Example P • Make an oxide of Phosphorous: – O needs 2 electrons P gives 3 • Simplest possible one is P2O3 (di)Phosphorous(III) trioxide • Next one up is: P2O5: (di)Phosphorous(IV) Pentoxide • Add water: P2 O3 + 3H 2O → 2 H 3 PO3 P2 O5 + 3H 2O → 2 H 3 PO4 – Phosphorous acid and Phosphoric acid • Make Salt: Replace H with Na – (tri)Sodium phosphite and sodium phosphate Na3 PO3 Na3 PO4 Summary Start learning these boldface ones. Determining Names and Formulas of Binary Covalent Compounds Problem 6-5: What are the name or chemical formulas of the following chemical compounds: a) carbon dioxide b) PCl3 c) Give the name and chemical formula of the compound formed from two P atoms and five O atoms. Solution: a) carbon dioxide b) PCl3 c) The compound formed from two P atoms and five O atoms Practice Problem 6-3 Determining Names and Formulas of Ionic Compounds Containing Polyatomic Ions a) magnesium perchlorate b) (NH4)2SO3 c) calcium nitrate d) Postassium permanganate Practice: Find these Metals in Periodic Table • Alkali metals - (lithium, sodium, potassium, rubidium and cesium) - soft, low melting points, react with water to liberate hydrogen, form 1:1 compounds with chlorine. • Alkaline earths - (beryllium, magnesium, calcium, strontium, barium and radium) - react in a 1:2 ratio with chlorine. (Type I in text) • Transition metals - (e.g. iron, copper, silver, gold, tungsten and cobalt) - structural metals. Multi-valence; (Type II in text) • Actinides and Lantanides: Often multi-valence • Metalloids - (antimony, arsenic, boron, silicon and tellurium) - intermediate between metals and nonmetals. Acts as metals with non-metals; Act as non-metals with metals. Answers to Some Problems in Lecture 1. (b) ZnCl2 , Zinc Chloride; (d) Sr3N2 , Strontium Nitride; (f) Sc2S3 , Scandium Sulfide 2. (b) Cobalt (II) Fluoride; (d) Nickel (III) Chloride 3. (a) Mg( ClO4)2 ; (b) Ammonium Sulfite; (c) Ca(NO3)2 4. (a) iodide, hydroiodic acid, HI; (c) sulfite, sulfurous acid, H2SO3 (e) cyanide, hydrocyanic acid, HCN 5. (a) CO2 ; (b) phosphorous trichloride; (c) diphosphorous pentaoxide NAMING OXOANIONS - EXAMPLES per hypo Root Suffixes “ ” ate “ ” ate “ ” ite “ ” ite Chlorine Bromine Iodine perchlorate perbromate periodate [ ClO4-] [ BrO4-] [ IO4-] No. of O atoms Prefixes chlorate [ ClO3-] bromate [BrO3-] iodate [ IO3-] chlorite [ ClO2-] bromite [ BrO2-] iodite [ IO2-] hypochlorite hypobromite hypoiodite [ BrO -] [ IO -] [ ClO -]