Covalent Nomenclature

Covalent Nomenclature
Naming Molecular Compounds
Review
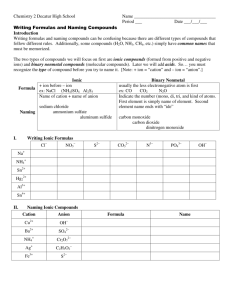
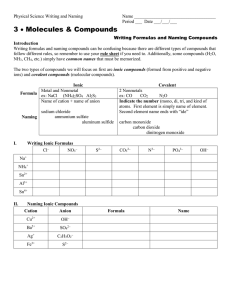
How do we identify ionic formulas?
Ionic formulas start with metals (or NH
4
+1 )
How do we identify covalent formulas?
Covalent formulas contain only non-metals or metalloids.
How do we name ionic compounds?
Identify the compound as binary or ternary.
Identify the metal as fixed charge or variable charge.
If the compound is binary, ends in –ide.
If the compound is ternary, name the anion appropriately.
Naming Molecular Compounds
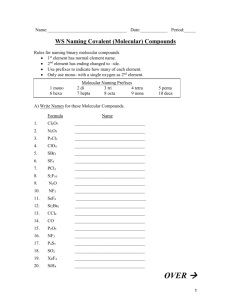
All the molecular compounds you’ll be asked to name are binary.
Molecular compounds w/ more than 2 elements are
usually organic.
Organic compounds have their own special naming system.
Atoms in molecules don’t the number of each atom:
mono = 1
hexa = 6
di = 2
hepta = 7
tri = 3
octa = 8
tetra = 4 penta = 5
nona = 9 deca = 10
Naming Molecular Compounds
Prefix for first element in compound.
Omit if it’s “mono”.
Name first element.
Prefix for second element in compound.
Give stem of second element followed by suffix –ide.
Examples:
CO = carbon monoxide
CO
2
= carbon dioxide
N
2
O = dinitrogen monoxide
Details and Exceptions
If the last letter of the prefix and the first letter of the element are both vowels, drop the last letter of the prefix.
CO = carbon monoxide, not carbon monooxide
N
2
O
5
= dinitrogen pentoxide, not dinitrogen pentaoxide
Some compounds are known only by their common names
H
2
O = water
NH
CH
4
3
= ammonia
= methane
Writing Formulas From Names
Write symbols in the same order they appear in the name.
Use prefixes to determine subscripts.
Metalloids are not metals.
Compounds that start with metalloids are named as molecular compounds.
Naming Molecular Compounds
What are the names of the following molecular compounds?
nitrogen dioxide
NO
2
SO
3
SiO
2
NO
P
2
O
5
S
4
N
4 sulfur trioxide silicon dioxide nitrogen monoxide diphosphorus pentoxide tetrasulfur tetranitride
Writing Formulas From Names
What are the formulas of the following molecular compounds?
carbon tetrachloride phosphorus trichloride selenium dibromide dinitrogen tetroxide sulfur dioxide xenon tetrafluoride diantimony trioxide
CCl
4
PCl
3
SeBr
2
N
2
O
4
SO
2
XeF
4
Sb
2
O
3