F11-Physics 231 lectures_29and crap
advertisement

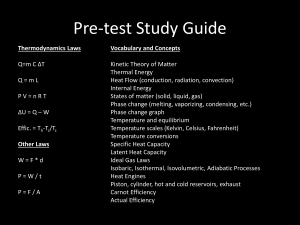
Physics 231 Lecture 29 • Main points of today’s lecture: • Temperature 5 Tcelcius (Tfarenheit 320 ) 9 Tcelcius Tkelvin 273.15 • Thermal expansion L TL; A A0 T ; V V0 T • Ideal gas properties: PV nRT Nk BT 2 N 1 2N 2 m v KE 3 V 2 3V 3 KE K E k BT 2 P Zeroth Law of Thermodynamics • If objects A and B are in thermal equilibrium with a third object, C, then A and B are in thermal equilibrium with each other. • Allows a definition of temperature Different Temperature Scales • Three different temperature scales are commonly in use: – Fahrenheit – Celsius – Kelvin • Celsius define such that the freezing point of water is at a temperature of about 0o C and boiling temperature is at about 100o C. • The conversions from Celcius to • At TK=0, molecules in gas are nearly at rest and many material have unusual properties. Farenheit and to Kelvin are as What is this temperature in Fahrenheit? follows: 0 273.15 C T T 273.15 0 C K a) 0 F 9 TF TC 32 9 b) -2730 F 5 TF TC 32 5 c) – 4600 F TK TC 273.15 9 0 273.15 32 459.670 F d) -923 F 5 Thermometers • While we know what is hot or cold. Much of what we think is simply perception: – Wind makes day “feel” colder because moving air strips away the insulating layer of warm air next to our skin. – Cold metal feels colder than equally cold wood. – Humid winter days feel colder than dry winter days. How do we construct a reliable thermometer? • Need a quantity that has a unique dependence on temperature. Example the length of a column of mercury in a thermometer grows linearly with temperature. • Many solids and liquids expand linearly with T: L L0 T • Example: A aluminum rod which is 1.0000 m long at 0C. What is its length at 100 C? (Al=23x10-6 / C) 6 o o a) 0.9989 m L L0 T 23x10 / C 1.0000m 100 C b) 1.0011 m L 0.0023m c) 1.0023 m L L0 L 1.0023m d) 1.0045 m Temperature and Thermal Expansion Slide 12-42 Area and Volume expansion • The areas and volumes of many objects also expand with temperature. • Example: Calculation of the area expansion coefficient for a rectangular aluminum plate. Af xy x0 x y0 y ; x x0 T ; y y0 T Af x0 x0 T y0 y0 T x0 y0 1 T 1 T Af A0 1 2T 2 T 2 A Af A0 2TA0 y x A TA0 where 2 • By analogy, the volume expansion coefficient =3 for a uniform material, where: V TV0 • Quiz: A swimming pool contains 110 m3 ( about 30,000 gal of water). The sun heats the water from 17 to 27C. What is the change in the volume of the water? =2.07x10-4 / C. – a) 0.08 m3 2.07x10-4 / o C V0 110m3 V V0 T – b) 0.13 m3 V 110m3 2.07x10-4 / o C 10o C 0.228m3 3 – c) 0.17 m – d) 0.23 m3 Example • A brass ring of diameter 10.00 cm at 20.0°C is heated and slipped over an aluminum rod of diameter 10.01 cm at 20.0°C. Assuming the average coefficients of linear expansion are constant, to what temperature must this combination be cooled to separate them? (Al=24x10-6 /oC, Br=19x10-6 /oC) Want D Br D Al D Br D Br,0 D Br ; D Al D Al,0 D Al D Br,0 D Br D Al,0 D Al D Br,0 D Al,0 D Al D Br 10.00 10.01 Al D Al T Br D Br T 0.01 A D Al Br D Br T 0.01 (24x10 6 10.01 19x10 6 10.00) T 5.0x10 5 T 0.01 0 0 199 C T C T 5 5.0x10 Tf 200 C T 1790 C The special case of Ice • Many material contract when changing from liquid to solid. Ice is an exception. The lowest energy configuration of the solid occupies a larger volume than the same mass of liquid. • The fact is key to spread of life across the planet because it means that ice is on the surface of large bodies of water where it will melt during summer rather than on the bottom. Phases of Matter In the solid form, ice, the molecules are farther apart than they are in the liquid form, water. Slide 12-16 Reading Quiz 2. A sample of nitrogen gas is in a sealed container with a constant volume. Heat is added to the gas. The pressure A. B. C. D. increases stays the same decreases can’t be determined with the information given Slide 12-8 Ideal gas • Ideal gas pressure depends linearly on temperature. nR TK V If T is measured in Kelvin P aT b • Ideal gas pressure depends linearly on temperature. • Here n=number of moles of the gas. There are NA = 6.02x1023 molecules or atoms (if the atoms don’t combine into molecules) per mole. One mole = .0224 m3 of gas at T=0oC and P=1 atm. • R=8.31 J/(moleK) • Example: A molecular gas is contained in an 8.0-L vessel at a temperature of 20°C and a pressure of 9.0 atm. (a) Determine the number of moles of gas in the vessel. (b) How many molecules are in the vessel? PV 9 1.01x105 Pa .008m3 a) n 3.0 moles RT 8.31J / K 293K b) N nN A 3 6.02x1023 1.8x1024 molecules Example • Gas is confined in a tank at a pressure of 10.0 atm and a temperature of 15.0°C. If half of the gas is withdrawn and the temperature is raised to 65.0°C, what is the ratio of the final density over the initial density? N0m 0 V 1 N0m Nf m f 2 V V 1 0 2 • What is the new pressure in the tank? N N0 Pf f k BTf k BT0 V V Nf k BTf N f Tf Pf 1 273 65 1 338 V 0.59 N 0 T0 P0 N 0 k T 2 273 15 2 288 V B 0 P0 Pf 0.59 10atm 5.9atm Physics 231 Lecture 30 • Main points of today’s lecture: PV nRT Nk BT • Ideal gas law: 2 N 1 2 N 3 2 P m v KE ; KE KE k BT 3 V 3 V 2 2 • Heat and heat capacity: Q cmT • Work in thermodynamic processes: Wsystem PV • First Law of Thermodynamics: U Q PV Checking Understanding: Pressure and Forces The two identical cylinders contain samples of gas. Each cylinder has a lightweight piston on top that is free to move, so the pressure inside each cylinder is equal to atmospheric pressure. One cylinder contains hydrogen, the other nitrogen. Both gases are at the same temperature. The number of moles of hydrogen is A. greater than the number of moles of nitrogen. B. equal to the number of moles of nitrogen. C. less than the number of moles of nitrogen. PV nRT n TH =TN VH =VN PH =PN nH/nN=? PV RT Slide 12-24 Checking Understanding: Pressure and Forces The two identical cylinders contain samples of gas. Each cylinder has a lightweight piston on top that is free to move, so the pressure inside each cylinder is equal to atmospheric pressure. One cylinder contains hydrogen, the other nitrogen. The mass of gas in each cylinder is the same. The temperature of the hydrogen gas is A. greater than the temperature of the nitrogen. B. equal to the temperature of the nitrogen. C. less than the temperature of the nitrogen. TH /TN=? VH =VN=V PH =PN=P mH=mN=m PV 1 PV T PV nRT n T n R n 1 nR H H H N m PV 1 TN n H 14 m n H 2g n N 28g n H m & n N 28g n NR n N Slide 12-26 2g Reading Quiz 2. When the temperature of an ideal gas is increased, which of the following also increases? (1) The thermal energy of the gas; (2) the average kinetic energy of the gas; (3) the average potential energy of the gas; (4) the mass of the gas atoms; (5) the number of gas atoms. A. B. C. D. E. 1, 2, and 3 1 and 2 4 and 5 2 and 3 All of 1–5 Slide 11-8 Reading Quiz What is the mass, in u, of a molecule of carbon dioxide, CO2? A. B. C. D. E. 12 24 32 36 44 Each nucleon has a mass of about 1 u. Most carbons has 6 protons and 6 neutrons for a total of 12 nucleons or m=12u. Most Oxygens have 8 protons and 8 neutrons for a total of 16 nucleons or m=16u. The total mass is 12+2*16 u = 44 u Slide 12-12 Kinetic properties of an ideal gas • Consider the gas volume at shown on the right. A molecule with mass m is elastically scattered by the wall which supplies an impulse force F1. F1t mv x m( v x ) 2mv x F1 wall 2mv x t wall • After a time t=2d/(vx), the mass bounce off the far wall and return to bounce of this wall again. If we use this value for t in the expression above, we can get the average force for much large time intervals. 2mv F1 x 2d / vx d A mv x2 / d • Multiplying by the number of molecules in the gas and dividing by the area A, we get the pressure on the wall. P N mv 2 N mv 2 Ad x V x • This would be correct if each molecule was moving the x direction with exactly velocity vx. To correct for the differences in velocities, we use the average <vx2> instead. Also <v2>= <vx2>+ <vy2>+ <vz2>=3 <vx2>. Thus” P N 2 N 1 2N 2 m v2 / 3 m v KE V 3 V 2 3V Average kinetic energy of gas molecules • If we combine the last expression with the ideal gas law equation of state, we get a useful expression for the means kinetic energy of gas molecules: 2N R n N P KE P RT N nN A k B P k BT 3V NA V V 2N N 1 1 3 KE k BT KE m v 2 mv 2rms k BT v rms 3V V 2 2 2 3k BT m note: increasing temperature increases thermal (KE) energy (adding heat energy Q) • Example: If the translational rms speed of water molecules (H2O) in a volume of air is 648 m/s, what is the translational rms speed of carbon dioxide (CO2)? Both gases are at the same temperature. (vrms[<v2>]1/2) v rms,H2O v rms,CO2 3k BT m H2O m H2O m CO2 v rms,H2O v rms,CO2 3k BT m CO2 N A m H2O N A m CO2 v rms,H2O m H2O 3k BT m CO2 m H2O m H2O m CO2 3k BT m H2O 2 16 648m / s 414m / s 12 2 16 quiz • Consider a gas mixture of the two monoatomic gases: 50% Helium (Mmole=4.0 g; Mmole is the mass of one mole of the gas.) and 50% Argon (Mmole=36.0 g). The ratio vrms,He / vrms,Ar of the rms speed for helium atoms divided by the rms speed for Argon atoms is: – a) .06 3k BT 3k BT – b) .17 v rms,Ar v rms,He m Ar m He – c) .67 – d) 1.3 – e) 3.0 v rms,He v rms,Ar 3k BT m He 3k BT m Ar 1 m He m He m Ar 1 m He m Ar m Ar m Ar 36 3 m He 4 Speed and Kinetic Energy of Gas Molecules 1 2 Kavg = mv rms <KE> 2 <KE> 2K avg T 3 kB 2 K avg 123kmv T vrms = B rms K avg 23 kmBT kB 1.38 10 23 J/K PV NkBT m F(v) 4 2 k BT 3/2 mv 2 v exp 2k T 2 B Slide 12-17 The Ideal Gas Model 2 <KE> Kavg T 3 kB 3 E th N KE Nk BT 2 Transfer of heat at constant volume: Slide 11-18 Checking Understanding Two containers of the same gas (ideal) have these masses and temperatures: • Which gas has atoms with the largest average thermal energy? • Which container of gas has the largest thermal energy? A. P, Q 1 3 2 mv k BT, which is independent of mass B. P, P 2 2 C. Q, P Q has the highest T highest thermal D. Q, Q energy per atom 3 E th Nk BT E th,P 2 E th,Q 3 N P k BTP 2 3 N Q k BTQ 2 N P TP 100 273 0 1 NQ TQ 20 273 50 Slide 11-21 Physics 231 Lecture 31 • Main points of today’s lecture: • Heat and heat capacity: Q cmT • Phase transitions and latent heat: Q Lm • Mechanisms of heat flow. • Conductive heat flow Q kAT2 T1 t L • Examples of heat conductivity, R values for insulators H Ri Li / ki Heat and heat capacity • We saw that for atoms or molecules in an ideal gas <KE>=3/2kBT. In general, the atoms or molecules in matter increase in energy if the object is heated. This is thermal energy, which can be transfer to and from the gas. • We define the heat energy Q to be the energy that flows from a hot object to a cold object solely because of the difference in T. • When the heat flow is sufficient, the two objects will reach the same temperature and we say they are in thermal equilibrium at the same T. • If we put Q into a mono-atomic ideal gas at constant volume: Q E th E th,f E th,i Q 3 3 3 Nk b Tf Nk b Ti Nk b Tf Ti 2 2 2 3 3 Nk b T nRT nc molar,V T cmolar,V=3/2R is the heat capacity per mole at 2 2 constant volume for mono-atomic ideal gas • For many other materials, the relationship between heat transferred and temperature change is given by: (usually transferred at constant V or P) Q cmT c is the specific heat capacity per unit mass m is the mass and cm the total heat capacity of the object. Example • Helium (He), a monoatomic gas, fills a 0.010 m3 container. The pressure of the gas is 6.2x105Pa. How long would a 0.25 hp engine have to run (1 hp=746 W) to produce an amount of work equal to the thermal energy of this gas? 3 3 E th N KE Nk BT nRT 2 2 PV nRT 3 3 E th Ppressure V 6.2x105 Pa 0.010m3 9300J 2 2 E th Work 0.25 746W t E 9300J t 50s 0.25 746W 187W Quiz • Two moles of the monatomic gas helium (CV=3/2R) are initially at a temperature of 300K. The gas is cooled at constant volume. How much heat must be removed to decrease the temperature (in Kelvin) by a factor of two? (Note: R=NAkB=8.31 J/(molek)) – a) 1.7 kJ 3 3 E th,f nRTf E th,i nRTi – b) 2.7 kJ 2 2 E th,f E th,i Q – c) 3.7 kJ – d) 4.7 kJ 3 3 1 Q E E nRT nRT th,f th,i Ti f i TF – e) 5.7 kJ 2 2 2 3 3 2moles 8.31J / mole K 150K Q nR Tf Ti 2 2 Q 3.7kJ removed energy = 3.7 kJ Conservation of heat energy • Imagine two objects A and B, with thermal energies Eth,A.0 and Eth,B,0 and TA>TB. • When they are put in thermal contact, they will come to equilibrium and heat Q will be transferred from A to B. Then, we can write E th,A,f E th,A,0 QA E th,B,f E th,B,0 QB From conservation of energy: QB QA 0 QB QA Q E th,A,f E th,A,0 Q E th,B,f E th,B,0 Q Example of equilibration and energy conservation • At a fabrication plant, a hot metal forging has a mass of 75 kg and a specific heat capacity of 430 J/(kg °C). To harden it, the forging is quenched by immersion in 710 kg of oil that has a temperature of 32°C and a specific heat capacity of 645 cal/(kg °C). The final temperature of the oil and forging at thermal equilibrium is 47°C. Assuming that heat flows only between the forging and the oil, determine the initial temperature of the forging. Qoil coil m oil Tf Toil Q metal c metal m metal Tf Tmetal Energy conservation: Q metal Qoil 0 Q metal Qoil 4.19J coil 645cal / (kg C) 2700kJ / (kg o C) cal o c metal m metal Tf Tmetal coil m oil Tf Toil Tf Tmetal coil m oil Tf Toil Tmetal Tf c metal m metal coil m oil Tf Toil c metal m metal mmetal 75 kg moil 710 kg cmetal 430J/kg/ oC coil 645cal/kg/ oC Tf 47 oC Toil,0 32 oC Tmetal,0 ? o o 2700J / (kg C) 710kg 47 32 C o 47 C 939 o C o 430J / (kg C) 75kg Example • Example: A 100 kg mass of water at a temperature of 30°C is dropped into a thermally isolated vessel containing 50 kg of water which is a temperature of 10°C. After system comes to thermal equilibrium the final temperature is (useful information: cwater=4186 J/(kg• °C)) – a) 11°C Q100 c water 100kg Tf 30o C – b) 14°C – c) 19°C Q50 c water 50kg Tf 10o C – d) 23°C Q100 Q50 0 c water 100kg Tf 30o C c water 50kg Tf 10o C 0 2 Tf 30o C Tf 10o C 0 Tf 23o C 3Tf 60o C 10o C Phase transitions T Q • Many substances display a plateaus in the caloric curve that describes T vs. Q. Regions with a linear increase correspond to a constant heat capacity where Q cmT T Q /(cm) • The flat regions occur when the system has a phase transition. For water, there is one where ice changes to water and other where water changes to steam. If the pressure remains constant during the phase change, the temperatures will remain constant. The heat required to change a mass m of the matter is given by the latent heat L for the phase change. Q Lm L fusion 33.5 x104 J / kg Lvaporization 22.6 x105 J / kg Example • When it rains, water vapor in the air condenses into liquid water, and the energy is released. (a) How much energy is released when 0.0254 m (one inch) of rain falls over an area of 2.59x106 m2 (one square mile)? (b) If the average energy needed to heat one home for a year is 1.5x1011 J, how many homes can be heated with the energy determined in part (a)? a) m water water Vwater 1000kg / m3 2.59x106 m 2 .0254m 6.58x107 kg Q released L vaporization m water 22.6x105 J / kg 6.58x107 kg 1.5x1014 J b) n houses 1.5x1011 J 1.5x1014 J n houses 1000 Heat flow • There are three major processes that transfer heat from one point to another. – Conduction – Convection – Radiation • Convection results from the fact that hot objects generally expand. This decreases their density. If this occurs in a fluid, the less dense hot fluid rises and the colder denser fluid falls. Conduction • Conduction concerns the transfer of heat through materials without convection. • Consider an concrete wall of a heated garage. The outside of the garage is at temperature T1 and the interior of the garage is a temperature T2. The conductive heat flow through a portion of the wall with area A is given by: H Q kAT2 T1 kAT t L L • H is the heat flow through the wall, k is the thermal conductivity of concrete, and L is the thickness of the concrete wall. • Example: Calculate the heat flow through a 2 m2 section of a 20 cm thick concrete wall when the outside temperature is 0 oC and the inside temperature is 20 oC. Assume the thermal conductive of the concrete is 1.3 J/(s m oC) 1.3J / (s m o C) 2m 2 20 o C Q kAT H 260W t L 0.2m Physics 231 Lecture 32 • Main points of today’s lecture: • Heat flow Q kAT2 T1 H t L • Examples of heat conductivity, R values for insulators Ri Li / ki • Convection • Radiation P AeT 4 where 5.67x10-8W /( A K 4 ) • Work in thermal processes: Wsystem PV Quiz • Two materials have the same insulating value if the same amount of heat per second per square meter flows through each due to the same temperature difference. Ignoring air convection, what thickness of body fat is required to give the same insulating value as a 0.010 inch thickness of air? (kfat=0.2 J/(sm oC), kair=0.023 J/(sm oC)) – – – – a) 0.09 inches b) 0.7 inches c). 2.3 inches d) 4.2 inches want H fat H air k fat AT k air AT Lfat Lair Lfat Lair k fat k air Lfat Lair k fat 0.2 0.01inch .09inch k air 0.023 Conceptual quiz • Two large metal blocks made of the same material and at the same temperature are brought together and placed into thermal contact. Block A contains twice as much heat as Block B. Which of the following statements is true – a) There will be net flow of heat from A to B – b) Net flow of heat from B to A – c) No net heat flow will occur – d) Not enough information to tell H Q kAT2 T1 kAT t L L Layered materials – R values Li • Consider the layered insulating structure at the right. The area of each layer is the same. Each layer can have a A different thickness Li and heat conductivity ki. • Here the important thing to remember is that we are concerned with a steady state solution. There is no build H up of heat in any of these layers. For each layer one has: Li Q k i ATi H Li H H Ti R i ; R i t Li A ki A ki • Here Ri it the “R value” of the ith insulating layer. The total temperature difference is given by the sum. i.e. T H T Ti R i A i i H Q AT AT t R i R tot i H Ri A i Example R Cu • Two rods, one of aluminum and the other of copper are joined end to end. The cross-sectional area of each is 4.0x10-4 m2, and the length of each is 0.04 m. The free end of the aluminum rod is kept at 302 oC, while the free end of the copper rod is kept at 25 oC. The loss of heat through the sides of the rods may be ignored. (a) What is the temperature at the aluminum-copper interface? (b) How much heat is conducted through the unit in 2.0 s? (kalum = 238 J/(s m oC), kCu = 397 J/(s m oC)) Do b) first. Q AT R tot R Cu R Al b) H t R tot LCu .04m 1.01x10 4 s m 2 C / J k Cu 397J / (s mC) R Al L Al .04m 1.68x10 4 s m 2 C / J k Al 238J / (s mC) } R tot 2.69x10 4 s m 2 C / J 4 2 AT 4x10 m 302 25 C H 412W Q Ht 412W 2s 824J 4 2 R tot 2.69x10 s m C / J 4 2 R Cu H LCu 1.01x10 s m C / J a) TCu =H TCu 412W 103.7C 4 2 A k Cu A 4x10 m Tinterface 25 o C 103.7 o C 128.7 o C Radiation • The Earth is warmed by the radiation from the sun. • The rate at which an object radiates energy is given by Stefan’s Law which provides the power radiated from a object with surface area A at temperature T. P AeT 4 where 5.67x10-8W /( A K 4 ) • Here e is emissivity (0e1) which describes how the radiation or absorption can be reduced below that for a “black” surface that absorbs light completely. • Example: The filament of a light bulb has a temperature of 3.0x 103 oC and radiates sixty watts of power. The emissivity of the filament is 0.36. Find the surface area of the filament. – a) 3.4 m2 60W P A 4 4 – b).063 m2 eT 5.67x108 W / (m 2 K 4 )(.36) 3273K – c) 2.6x10-5 m2 5 2 A 2.6x10 m -6 2 – d) 9.8x10 m Example • A solid aluminum sphere is coated with lampblack (emissivity=0.97) and hung inside an evacuated container. The sphere has a radius of 0.020 m and is initially at 20.0 oC. The container is maintained at a temperature of 70.0 oC. (a) Assuming that the temperature of the sphere does not change very much, what is the net energy gained by the sphere in 10.0 s? (b) Estimate the change in temperature of the sphere. (Note: all objects radiate and absorb radiation at the same time.) a) Detailed balance: In equilibrium power emitted power absorbed. P20 Ae (293K) 4 power emitted P70 Ae (343K) 4 power absorbed E P70 P20 t is energy gained by sphere E Ae P70 P20 t 5.67x10-8 4 (0.02) 2 (.97)[ 343 293 )10s 18J 4 4 b) Have E Q (no work), need heat capacity to get temperature change ΔE T ΔE calum m alum T calum alum Valum T 3 calum alum 4 .02m / 3 18J 0 T 0.22 C 3 3 (900J / (kg K)) 2700kg / m 4 .02m / 3 Temperature of the Planets • The surface temperature of the Sun is about Planet 5800 K. a) Taking the Sun’s radius to be 6.96 x Mercury 108 m, calculate the total energy radiated by the Venus Sun each second. (Assume e = 0.965.) b) Earth Calculate the equilibrium temperature of the Earth, Mercury, Venus and Mars. Assume for Mars simplicity that the emissivity of the Earth, Mercury, Venus and Mars are all also 0.965. The orbital radii of these planets are given in the table. Orbital radius ~5.8x1010 m 1.1x1011 m 1.5x1011 m 2.3x1011 m First calculate the radiant power emitted by the sun 4 a) Psun AeTsun 5.67x10 8 W / (m 2 K 4 )4 6.96x108 m Psun 3.76x1026 W 2 .9655800K 4 Temperature of planets continued • Let’s calculate for the earth first. At a distance equal to the earth’s radius, rearth, Psun is spread over a spherical area of 4r2earth.. The earth covers a circular area of R2earth where Rearth is the radius of the earth. • The power absorbed on earth therefore is: 2 eearthRearth b) Psun _ on _ earth Psun 2 4rearth • The earth is at temperature Tearth and radiates in all directions with a power: P A e T 4 4R 2 e T 4 earth earth earth earth earth earth earth In steady state Psun _ on _ earth T 4 earth 2Re 2 eearthRearth 2 4 Psun P 4 R e T earth earth earth earth 2 4rearth Psun re 2 eearthRearth Psun 9 4 5 . 88 x 10 K 2 2 2 4rearth 4Rearth eearth 16rearth Tearth 277 K 00C 4 Note : Tmerc . Psun Psun 4 T venus 2 2 16rmerc 16rvenus . 4 Tmars Psun 2 16rmars Tmerc. 445 K 1720C Tvenus 323K 500C Tmars 223K 500C Sometimes Planet Orbital radius Calculated T Measured <T> Mercury 5.8x1010 m 445 K 443K Venus 1.1x1011 m 323 K 723 K Earth 1.5x1011 m 277 K 287 K Mars 2.3x1011 m 223 K 230 K • Even though Mercury is much closer to the Sun than Venus is, Venus is the hottest planet in our Solar System. Temperatures on the plains of Venus often reach 850 degrees Fahrenheit (450 degrees Celsius). This is because of the thick layer of carbon dioxide clouds that surround Venus. Much of the heat from the Sun that hits Mercury escapes back into space because Mercury does not have a large atmosphere with clouds to trap in the warmth. But the carbon dioxide clouds on Venus are so thick they create what scientists call a Greenhouse Effect. • We cannot calculate the Greenhouse effect using Stefan’s equation. It occurs because the emissivity of the Venusian atmosphere is much larger for long wavelength infrared light and smaller for shorter wavelength light. Physics 231 Lecture 33 • Main points of today’s lecture: • Work in thermodynamic processes: Wsystem PV • First Law of Thermodynamics: U Q PV • Specific heat at constant pressure • Processes P V T 0 – cyclic: – isobaric: P 0 – isovolumetric: V 0 T 0 – isothermal: Q 0 – adiabatic: Conceptual question • Which gives the largest average radiation absorbed on a 1 m2 area at that distance? – 1. a 50-W source at a distance R. – 2. a 100-W source at a distance 2R. – 3. a 200-W source at a distance 4R. Pabsorbed fraction of area covered e emitted power Pabsorbed 1m2 eP 2 4 r 1m2 P1 e 50 2 4 R 1m2 P2 e 100 2 4 (2R) 1m2 P2 e 200 2 4 (4R) Work and the 1st Law of thermodynamics • • • Consider the ideal gas contained in the volume under the cylinder at right. The piston compresses the gas very slowly, moving downwards with a constant velocity. The gas exerts a force Fgas=PA upward on the piston and conversely, the piston exerts a force Fpiston=-PA downward on the gas. The work done by the piston on the gas is: W piston Fpiston y PA y PV • The work done by the gas on the piston is: Wgas Fgas y PA y PV • If U denotes the internal energy of the system, the conservation of energy dictates: U E th Q Wpiston Q Wgas Q PV 1st Law of Thermodynamics Here I have changed notation to U, because other systems, besides the ideal gas, can have potential energy. Why does work increase thermal energy • Recall that the thermal energy U is given by U=3/2NkBT • If work done on the gas increases U it must increase T. • Positive work done on the gas increases U because the collisions with the piston moving downward increases the velocity of the molecules. • Negative work done on the gas decreases U because the collisions with the piston moving upward decreases the velocity of the molecules. Example • The work done to compress one mole of a monatomic ideal gas is 6200 J. The temperature of the gas changes from 350 K to 550 K. How much heat flows between the gas and its surroundings? Determine whether the heat flows into or out of the gas. Work done by the gas is : Wgas 6200J 3 Internal energy change in the gas is: U nR T 2 3 3 Q U Wgas nR T 6200J 8.31J / K 550 350K 6200J 2 2 Q 3700J Computation of work: • What is the work done by the gas going from (Pi ,Vi) to (Pf ,Vf) ? – a) Pi(Vi-Vf) – b) Pi(Vf-Vi) – c) 0 – d) Vi(Pi-Pf) – e) Vf(Pi-Pf) • The work done by the gas is the mathematical area under the curve Pi(Vf-Vi). It is negative if the volume is decreasing. It is pushing to increase the volume, but the volume is being decreased. • No work is done on the isovolumetric (constant volume) part of the path! Computation of work: • What is the work done on the gas going from (Pi ,Vi) to (Pf ,Vf) ? – a) Pi(Vi-Vf) – b) Pi(Vf-Vi) – c) 0 – d) Vi(Pi-Pf) – e) Vf(Pi-Pf) • Note that the work done on the gas is (-1) times the mathematical area under the curve, -Pi(Vf -Vi). The mathematical area is negative if the volume is decreasing. The work done on the gas is positive, you are compressing it and thereby exerting a force in the direction of that it is being compressed. • No work is done on the isovolumetric (constant volume) part of the path! Conceptual quiz • A gas is taken from an initial state of pressure and volume, PA VA,to a final, different, state, PB VB. As it changes work is done on the gas. – a) The amount of work depends on the path taken from A to B – b) The amount of work does not depend on the path taken from A to B quiz: • What is the work done on the gas going from (Pi ,Vi) to (Pf ,Vf) ? – a) Pf(Vi-Vf) – b) Pf(Vf-Vi) – c) 0 – d) Vi(Pi-Pf) – e) Vf(Pi-Pf) • Note that the work done on the gas is the negative of the mathematical area under the curve. The mathematical area is negative if the volume is decreasing. We are compressing the gas and the volume is decreasing and we are pushing in the direction to decrease it. • No work is done on the isovolumetric part of the path! Computation of work: • What is the work done on the gas going from (Pi ,Vi) to (Pf ,Vf) ? – a) Pi(Vi-Vf) – b) Pf (Vi-Vf) – c) 0 – d) (Pi+ Pf)(Vi-Vf)/2 – e) -(Pi+ Pf)(Vi-Vf)/2 Heat capacity at constant pressure • Consider a cylinder filled with a n=2 moles of monatomic gas and plugged at the top by a frictionless piston. Above the piston is atmospheric pressure. This ensures the piston will be at a constant pressure P=299kPa. The bottom of the cylinder piston is a heat source that can heat the gas. The sides of the cylinder and the piston have zero thermal conductivity. What is the heat Q required to raise the temperature of the gas from initial temperature Ti =300 K to final temperature Tf =400K? Heat source Q U W 3 U n RT 2 W pV 3 n R Tf Ti 2 p Vf Vi pVf pVi nRTf nRTi nR Tf Ti 3 5 Q n R Tf Ti nR Tf Ti n R Tf Ti 2 8.31*(400 300)J 1.66kJ 2 2 3 5 c p c v R R R R is the molar heat capacity at constant pressure 2 2 Example • A person takes in a breath of 0°C air and holds it until it warms to 37.0°C. The air has an initial volume of 0.600 L and a mass of 7.70 x 10–4 kg. Determine (a) the work done by the air on the lungs if the pressure remains constant at atmospheric pressure, (b) the change in internal energy of the air, and (c) the energy added to the air by heat. Model the air as if it were a diatomic gas: cV =5/2R., cp=7/2R. Pf Pi Patm 1.01x105 Pa Wgas Patm Vf Vi nR Tf Ti nRTf Tf Patm Vf 1 Patm Vi 1 Patm Vi 1 Patm Vi nRTi Ti Patm Vi 310 Wgas 1.01x105 Pa .0006m3 1 8.2J 273 Assume diatomic gas: T 5 5 5 U nR Tf Ti nRTi f 1 Wgas 20.5J 2 2 2 Ti 7 Q U Wgas nR Tf Ti 28.7J 2 Physic 231 Lecture 34 • Main points of today’s lecture: • Cycles • Reversible and irreversible processes. • Carnot cycle and Carnot engine. e Weng Qh Th Tc Th 1 – T is in Kelvin. • Engines and refrigerators. • Entropy: Q reversible S T Tc Th Thermal systems • In thermal systems, there are a set of “state variables” that are sufficient to completely describe the macroscopic state of the system. – example: ideal gas (P,T,N), (V,T,N), (P,U,N) and (U,V,N) are examples of a set of state variables. U=3/2NkBT is the internal energy. • Zeroeth law of thermodynamics: Two macroscopic systems are in thermal equilibrium if and only if they are at the same temperature. • In a thermal process, a macroscopic system changes its state variables in a smooth and controlled manner. Examples of some common processes are: – Isobaric process: P=0; the pressure remains the same – Isovolumetric process: V=0; the volume remains the same. – Adiabatic process: Q=0; the system is thermally isolated. – Isothermal process: T=0; the temperature remains the same. Additional Questions When I do work on a gas in an adiabatic process, compressing it, I add energy to the gas. Where does this energy go? A. The energy is transferred as heat to the environment. B. The energy is converted to thermal energy of the gas. C. The energy converts the phase of the gas. Slide 12-56 Cyclic process: • A thermal path which returns to its initial condition is called a cycle. • The work done by the gas on a clockwise cycle is the area contained in the path. • The work done by the gas on a counterclockwise cycle is the negative of the area in the path. • The work done on the gas is the negative of the work done by the gas. Example of Cyclic process • • • • The drawing refers to one mole of monatomic ideal gas and shows a process that has four steps, two isobaric (A to B and C to D) and two isovolumetric (B to C and D to A). a) Complete a table by calculating U, W and Q (including the algebraic signs) for each of the four steps. b)What is the net heat/cycle absorbed by the system ? c) What is the net work/cycle done by the system? n 1 3 3 3 RTA = (8.31)400J 4986J U B RTB 9972J 2 2 2 3 3 U C RTC 4986J U D RTD =2493J 2 2 U A B U B U A UA B 9972 4986 5.0kJ UA Path A-B U W W Q Q 5.0kJ 3.3kJ 8.3kJ B-C C-D - 5.kJ 0 - 5.kJ D-A 2.5kJ 0 2.5kJ similarly get U BC , UC D , & U D A Net 0 1.6 kJ 1.6 kJ WD A WBC 0 because isovolumetric PV nRT -2.5kJ -1.7kJ -4.2kJ WAB PA VB VA PB VB PA VA RTB RTA 3.3kJ WC D RTD RTC 1662J Q U W Important Cyclic Processes: Engines • In a heat engine, thermal energy Qh is used to do work, Weng. Some of the original thermal energy Qc escapes and ends up heating something else • A heat engine involves some working substance in a cyclical process. • In many cases heat comes from reservoir at TH and is exhausted to the invironment at TC. • Thermal efficiency is defined as the ratio of the work done by the engine to the energy absorbed at the higher temperature. For simplicity both can be computed over one cycle: e Weng Qh Qh Qc Qc 1 Qh Qh • e = 1 (100% efficiency) only if Qc = 0 – No energy expelled to cold reservoir, which is theoretically possible for Tc=0, but practically impossible. Example • The energy absorbed by an engine is three times as large as the work it performs. (a) What is its thermal efficiency? (b) What fraction of the energy absorbed is expelled to the cold reservoir? a) Q h 3W e W W 1 Q h 3W 3 b) Q h W Qc Qc Q h W 2W Qc 2 Qh 3 2 Qh 3 Quiz • A heat engine performs 200 J of work in each cycle and has an efficiency of 30%. For each cycle of operation, (1) how much energy is taken in from the hot reservoir (Qh) and (2) how much energy is expelled to the cold reservoir (QC)? • Answers below are in the form Qh, Qc. – a) 667J, 467J – b) 467J, 667J – c) 60J, 140J – d) 140J, 60J a) e W W 200 J Qh 667 J Qh e 0.3 b) QH W Qc Qc Qh W 667 J 200 J 467 J Reading Quiz 1. A sample of nitrogen gas is inside a sealed container. The container is slowly compressed, while the temperature is kept constant. This is a ________ process. A. B. C. D. constant-volume isobaric isothermal adiabatic Slide 12-6 Reversible and Irreversible Processes • reversible process is one in which every state along some path is an equilibrium state. – And one for which the system can be returned to its initial state by going along the same path in the (p,V) diagram but in the opposite direction. – Volume and pressure changes are “slow”. – When objects are brought into thermal contact, they are at the same temperatures. – Carnot cycle is an example of a reversible process. • An irreversible process does not meet these requirements – Most natural processes are irreversible • Burning fueling in an automobile engine • Dropping ice into warm water • Heating water on a range • Reversible process are an idealization, but some real processes are good approximations. Constant-Volume Process: Isovolumetric – Isovolumetric process: V=0; the volume remains the same, internal energy changes, heat can be exchanged with environment, but no work is done. Slide 12-29 Constant-Pressure Process – Isobaric process: P=0; the pressure remains the same, work can be done, heat can be exchanged and internal energy can change. Constant-Temperature Process: isothermal – Isothermal process: T=0; the temperature remains the same. Internal energy of a gas remains the same, heat can be exchanged and work can be done. nRT P V Adiabatic process: no exchange of heat with environment – Adiabatic process: Q=0; the system is thermally isolated. Heat cannot come in or out of the system, internal energy can change and work can be done. . . PV const an t 5 / 3 for monatomic ideal gas When a gas expands, it does work, when a gas is compressed, work is done on it. In an adiabatic process, the work done by a gas while expanding decreases its thermal energy and temperature. Conversely, the work done on the gas while compressing it increases its thermal energy and temperature. Operation of a Heat Engine Slide 11-26 Carnot Engine • A Carnot engine is the most efficient possible engine that takes heat from a hot reservoir at temperature Th and expells heat into a cold reservoir at temperature Tc . – It uses gas as the working substance. – It absorbs heat Qh during an isothermal expansion while in contact with the hot reservoir. – It expands adiabatically a little further. – It compresses isothermally while depositing QC into the cold reservoir. – It compresses adiabatically a little further. Carnot Cycle, A to B • A to B is an isothermal expansion • The gas is placed in contact with the high temperature reservoir • The gas absorbs heat Qh • The gas does work WAB in raising the piston • What happens to the internal energy of the gas? – a) It increases – b) It decreases – c) It stays the same • What happens to the pressure? – a) It increases – b) It decreases – c) It stays the same nRT P V Fig. 12.13, p. 374 Slide 22 Carnot Cycle, B to C • B to C is an adiabatic expansion • The base of the cylinder is replaced by a thermally insulating wall • No heat enters or leaves the system • The temperature falls from Th to Tc PV const an t • The gas does work WBC 5 / 3 for monatomic ideal gas • What happens to the pressure? – a) increases – b) decreases – c) stays the same • What happens to the internal energy? Q U pV 0 – a) increases – b) decreases – c) stays the same Fig. 12.13, p. 374 Slide 22 Carnot cycle efficiency • The efficiency of the Carnot cycle depends only on the temperatures of the hot and cold reservoirs: e Weng Qh Th Tc T 1 c Th Th – No engine operating between these two temperatures is more efficient than the Carnot engine • A heat engine operates between two reservoirs at temperatures of 20°C and 300°C. What is the maximum efficiency possible for this engine? • a) .24 TC 293 e e 1 1 0.49 • b) .49 max carnot TH 573 • c) .56 • d) .73 Carnot Cycle, C to D • The gas is placed in contact with the cold temperature reservoir • C to D is an isothermal compression • The gas expels energy QC • Work WCD is done on the gas • What happens to the internal energy of the gas? – a) It increases – b) It decreases – c) It stays the same • What happens to the pressure? – a) It increases – b) It decreases – c) It stays the same Fig. 12.13, p. 374 Slide 22 Carnot Cycle, D to A • D to A is an adiabatic compression • The gas is again placed against a thermally nonconducting wall – So no heat is exchanged with the surroundings • The temperature of the gas increases from Tc to Th • The work done on the gas is WCD • What happens to the pressure? – a) increases – b) decreases – c) stays the same • What happens to the internal energy? – a) increases – b) decreases – c) stays the same Fig. 12.13, p. 374 Slide 22 Example • An engine does 20900 J of work and rejects 7330 J of heat into a cold reservoir at 298K. What is the smallest possible temperature of the hot reservoir? a) Q h W Qc 20900J 7330J 28230J W 20900J e 0.74 Q h 28230J ecarnot Tc 1 Th Tc T c 1 .74 .26 Th Th Tc Tc 298K Th .26 Th .26 Th .26 0.74 1 1146K Th If Th were less than 1146, then the calculated efficciency 0.74 is larger than that of a Carnot engine running at that temperaure, which is impossible. Additional Questions Suppose you have a sample of gas at 10°C that you need to warm up to 20°C. Which will take more heat energy: raising the temperature while keeping the pressure constant or raising the temperature while keeping the volume constant? A. It takes more energy to raise the temperature while keeping the volume constant. B. It takes more energy to raise the temperature while keeping the pressure constant. C. The heat energy is the same in both cases. If you raise the temperature and keep the pressure constant, the gas will expand and do positive work. Q U W Slide 12-54 Additional Questions When I do work on a gas in an adiabatic process, compressing it, I add energy to the gas. Where does this energy go? A. The energy is transferred as heat to the environment. B. The energy is converted to thermal energy of the gas. C. The energy converts the phase of the gas. Q U pV 0 Slide 12-56 Quiz • A heat engine operating between a hot reservoir at 500 K and a cold reservoir at 200 K has an efficiency that is 70% of its maximum possible value. If it receives lx106 J heat energy from the hot reservoir in 25 minutes, it can do a quantity of work equal to – a)6.3x105J. Tc 200 – b)4.2x105J. a) e max ecarnot 1 1 0.6 Th 500 – c)3.lxl05J. – d)2.5x105J. e 0.7e max 0.7(0.6) .42 5 – e)1.7x10 J. W e Qh W e Q h .42 1x106 J 4.2x105 J Heat pumps and refrigerators • Heat engines can run in reverse – Send in energy – Energy is extracted from the cold reservoir – Energy is transferred to the hot reservoir • This process means the heat engine is running as a heat pump – A refrigerator is a common type of heat pump – An air conditioner is another example of a heat pump – In the south, people often use heat pumps to heat homes • A standard measure of the performance of a refrigerator is its “coefficient of performance” coef. of performance = QC W heat removed per cycle work required to remove it A Carnot refrigerator is a Carnot engine run in reverse • A Carnot refrigerator maintains the food inside it at 276 K while the temperature of the kitchen is 298 K. The refrigerator removes 3.00x 104 J of heat from the food. How much heat is delivered to the kitchen? As a Carnot heat engine, we know that Qc Tc Qc Tc ecarnot 1 1 Q h Th Qh Th The Carnot engine run in reverse takes mechanical energy W to move Qc from the inside of the refrigerator and deposit Q h in the kitchen. Qc Tc Th 4 298 Q h Qc 3x10 J 3.23x104 J Q h Th Tc 276 Note: Carnot coefficient of performance= QC QC TC W Qh Qc Th TC Checking Understanding: Increasing Efficiency of a Heat Pump Which of the following changes would allow your refrigerator to use less energy to run? (1) Increasing the temperature inside the refrigerator; (2) increasing the temperature of the kitchen; (3) decreasing the temperature inside the refrigerator; (4) decreasing the temperature of the kitchen. A. All of the above B. 1 and 4 C. 2 and 3 Assume the temperature dependence is similar to that of a carnot refrigerator, where Q h Th QC Tc Carnot Coefficent of performance = Tc Th Tc This is largest when Th and TC are closer to each other in temperature. Slide 11-31 Qreversible S T • Entropy can only be calculated from a reversible path, and must be done that that way even if the system actually follows an irreversible path – To calculate the entropy for an irreversible process, model it as a reversible process • When heat energy is absorbed, Q is positive and entropy increases • When heat energy is expelled, Q is negative and entropy decreases • In an adiabatic process Q=0 and entropy remains the same. • S ln(probability). • A disordered state with energy and matter spread out everywhere is more probable than having all of the energy stored in an organized way that can be used to do work. Example • The surface of the Sun is approximately at 5700 K, and the temperature of Earth’s surface is approximately 290 K. What entropy change occurs when 1000 J of energy is transferred by heat from the Sun to Earth? The sun loses Q of heat and therefore decreases its entropy by the amount Q Ssun Tsun The earth gains Q of heat and therefore increases its entropy by the amount Q Searth Tearth The total entropy change is: S Ssun Searth Q 1 1 1000 J 1 1 3.27J / K T 290 K 5700 K T earth sun S T Example • What is the change in entropy of 1.00 kg of liquid water at 100°C as it changes to steam at 100°C? Q S T L vap m T 22.6x10 J / kg 1kg 6x10 J / K 5 3 373K Entropy Higher entropy states are more likely. Systems naturally evolve to states of higher entropy. Slide 11-33 Second Law of Thermodynamics Slide 11-34 Example • A power plant has been proposed that would make use of the temperature gradient in the ocean. The system is to operate between 20.0°C (surface-water temperature) and 5.00°C (water temperature at a depth of about 1 km). (1) What is the maximum efficiency of such a system? (2) If the useful power output of the plant is 75.0 MW, how much energy is absorbed per hour? (3) In view of your answer to (1), do you think such a system is worthwhile (considering that there is no charge for fuel)? • Answers for 1 and 2: 278 Tc – a) .025, 3.2x106 J a) e e 1 0.051 max carnot 1 12 293 Th – b).051, 5.3x10 J – c) . 25, 3.2x107 J b) e W Q W 75MW 3600s 5.3x1012 J h e 0.051 Qh – d) 051, 5.3x1013 J c) What is the energy required to pump the water? Story of Hawaiian deep water project • • Keahole sits at a point where underwater • land slopes sharply down into the sea, it was a place where warm water can be piped from the surface of the sea and cold water can be piped from depths of about a half-mile. • A process called ocean thermal energy conversion, or OTEC, used the temperature difference between hot and cold sea water to produce 50 KW of electricity at Keahole in 1993. The process worked but it was uneconomical. KAILUA, HAWAI'I — Koyo USA Corp., a company selling deep-sea water from Keahole Hawai'i, is expanding its plant and has applied to sell the water in the United States The company is producing more than 200,000 bottles a day and says it can't keep up with demand in Japan, where it sells 1.5 liter bottles of its MaHaLo brand for $4 to $6 each. Physics 231 Lecture 23 • Main points of today’s lecture: • Springs and masses • Simple harmonic motion of a spring: x A cos(t 0 ) v x A sin(t 0 ) a x 2 A cos(t 0 ) 2 k / m 0 and A are constants • Pendulum: L T 2 g max cos2ft 0 Final Exam • • • • A common final exam time is scheduled for all sections of Physics 231 Time: 3pm-5pm, Thursday, May 5 . Location : E100 Veterinary Medical Center. This information can also be found on our course schedule page • An alternate exam time will be scheduled for students who have conflicts with the regular time. – Three students have confirmed conflicts with me and will take the exam then. – You must contact me by email and obtain permission from me to take the exam at the alternate time. • Alternate time: TBA (probably sometime Wed. May 4th) • Location: TBA Example • The surface of the Sun is approximately at 5700 K, and the temperature of Earth’s surface is approximately 290 K. What entropy change occurs when 1000 J of energy is transferred by heat from the Sun to Earth? The sun loses Q of heat and therefore decreases its entropy by the amount Q Ssun Tsun The earth gains Q of heat and therefore increases its entropy by the amount Q Searth Tearth The total entropy change is: S Ssun Searth Q 1 1 1000 J 1 1 3.27J / K T 290 K 5700 K T earth sun Qreversible S T Second Law of Thermodynamics Slide 11-34 Reading Quiz 1. The type of function that describes simple harmonic motion is A. B. C. D. E. linear exponential quadratic sinusoidal inverse Slide 14-6 Reading Quiz 2. A mass is bobbing up and down on a spring. If you increase the amplitude of the motion, how does this affect the time for one oscillation? A. The time increases. B. The time decreases. C. The time does not change. Slide 14-8