Thermal Physics Review Sheet: Key Concepts & Calculations
advertisement

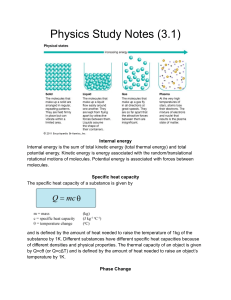
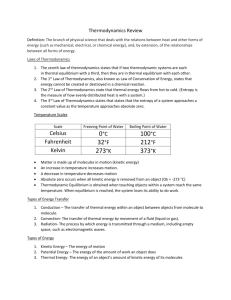
Review Sheet – Thermal Physics 1. Read chapters 10 and 11. 2. Terms to know: average kinetic energy, heat, thermodynamics, thermal energy, thermal equilibrium, absolute zero, Celsius temperature, Kelvin temperature, phase change, change of state, heat of fusion, heat of vaporization, condensation, melting, conduction, convection, radiation, calorimetry. 3. Define: a) entropy b) specific heat capacity c) latent heat capacity d) internal energy e) temperature f) thermal energy 4. State: The First Law of Thermodynamics 5. State: The Second Law of Thermodynamics 6. What are three methods of heat transfer? 7. In what direction will thermal energy transfer? When will it stop transferring? 8. State the formula for converting between Celsius and Kelvin. 9. State the variable and units for each of the following. State whether they are vector or scalar. a) thermal energy b) internal energy c) temperature d) work e) specific heat capacity f) latent heat capacity 10. State what happens to the temperature, the internal potential energy, the internal kinetic energy, and the total internal energy during each of the following processes: a) water heats up b) ice melts c) steam condenses d) steam heats up 11. Compare a cup of coffee and an iceberg. Which has more: a) temperature? b) average kinetic energy? c) total internal kinetic energy? d) internal energy? 12. Be able to calculate the heat required for a phase change, and the heat of vaporization and fusion for a substance. What formula is used? 13. Be able to calculate the specific heat of a substance and the heat necessary for a temperature change. What formula is used? 14. Give examples of changing the internal energy of a substance by heat and work. 15. Give an example of a natural process that increases the entropy of a system. 16. Be able to calculate the specific heat of a substance involved in a calorimetry experiment. What equation is used?