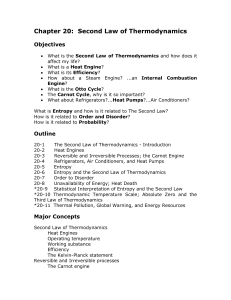
Thermodynamics Laws
Q=m C ∆T
Q = m L
P V = n R T
∆U = Q – W
Effic. = T h
-T c
/T c
Other Laws
W = F * d
P = W / t
P = F / A
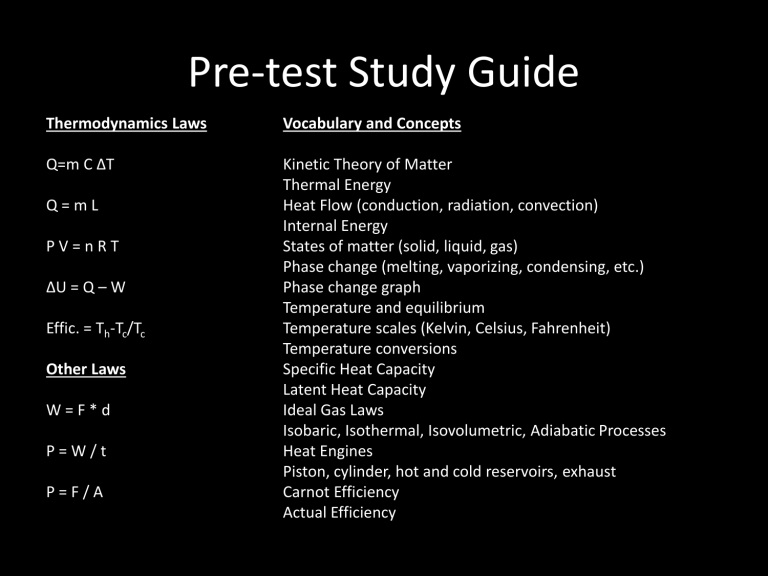
Pre-test Study Guide
Thermodynamics Laws Vocabulary and Concepts
Kinetic Theory of Matter
Thermal Energy
Heat Flow (conduction, radiation, convection)
Internal Energy
States of matter (solid, liquid, gas)
Phase change (melting, vaporizing, condensing, etc.)
Phase change graph
Temperature and equilibrium
Temperature scales (Kelvin, Celsius, Fahrenheit)
Temperature conversions
Specific Heat Capacity
Latent Heat Capacity
Ideal Gas Laws
Isobaric, Isothermal, Isovolumetric, Adiabatic Processes
Heat Engines
Piston, cylinder, hot and cold reservoirs, exhaust
Carnot Efficiency
Actual Efficiency
MARCH 5 TH , 2012
Thermodynamics Review
Material Freezing
Point
Boiling
Point
Water 273*K
Ammonia 195 *K
Ethanol 159*K
373*K
240 *K
352 *C
Solid Specific
Heat Capacity
(C s
)
2.11 J/g *K
? (use 4.6)
2.41 J/g *K
Liquid Specific
Heat Capacity
(C s
)
4.18 J/g *K
4.6 J/g *K
2.43 J/g *K
Gas Specific
Heat Capacity
(C s
)
2.08 J/g*K
2.17 J/g *K
1.70 J/g * K
Latent Heat of Fusion (Lf)
334 J/g
339 J/g
109 J/g
Latent Heat of
Vaporization
(Lv)
2260 J/g
1369 J/g
838 J/g
16) Using the information above, create an accurate phase change graph for
Ethanol.
17) A mixture of 50g of ethanol and 50g of water start at 20*C and are heated until they’re at 120*C. How much energy is consumed in this process?
18) A gas undergoes isobaric compression. The temperature goes from 200*K to
600*K during the process. How much of its original volume does the gas now occupy?
19) An engine has a piston area of 0.1 m2 and a piston volume of 0.02 m3. It is pushed forwards at a pressure of 50,000 Pa and then has to overcome a pressure of 10,000 Pa as it returns back to its original position. How much net work is done per cycle? If it cycles 120 times per minute, how much power does it generate?
20) An engine has a hot reservoir at a temperature of 100*C and a cold reservoir of
20*C. What is the theoretical maximum (Carnot) efficiency it can achieve? If it does 100J of work for every 500J of fuel, what is its actual efficiency?