Thermal Physics Summative Assessment Review Guide
advertisement

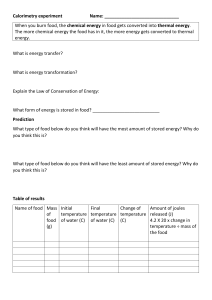
Thermal Physics Summative Assessment Review Guide You can expect 10 Multiple Choice Questions dealing with the following topics: - Particle motion in solids, liquids and gases - Power, energy and heat - Properties of an ideal gas - Specific latent heat of fusion and vaporization - Thermal equilibrium and energy transfer - Specific heat capacity - Definition of a mole - Internal energy and temperature - Calorimetry The rest of your test will be written questions from the following topics: - Internal energy and heating up of objects - Specific heat capacity questions and calculations - Molecular model of an ideal gas - Pressure - Molecular model of the different phases of matter (solid liquid, liquid gas, gas liquid, liquid solid) - Evaporation and boiling - Topics from Mechanics (Unit 2) that are related to Thermal Physics Remember, your final exam will have a mixture of unit questions in each question. As we go further in our studies you will be expected to make connections with previous material. Think about what we have covered and how it could be related to topics from the past. For instance, heat is measured in Joules. Work, kinetic energy, potential energy are also measured in Joules; there is a connection there. Work is force x displacement, so forces could be involved too. Force = mass x acceleration; so the kinematics equations of motion may be useful to review also. Please remember that you will have 5 minutes to read your test and an additional time to draw boxes around each answer. Your test will also be timed to mark the exact amount of time you would have on an IB test to answer these questions. Good luck studying and I am available on Monday if you have any questions.