Problem Set 4 - 2004
advertisement
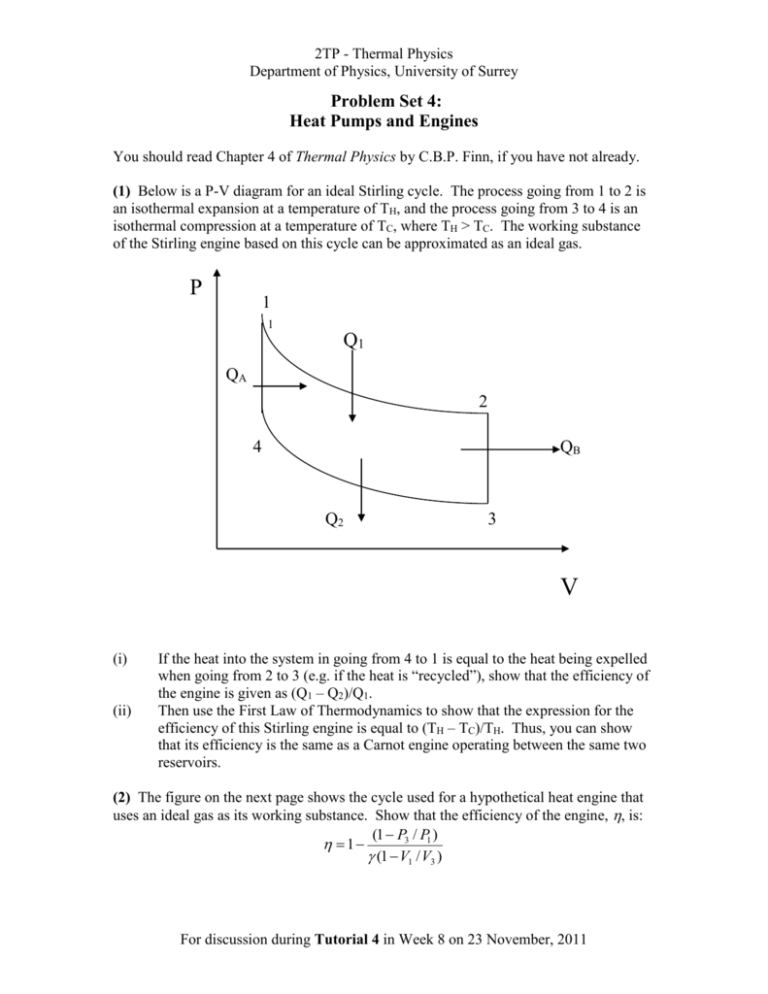
2TP - Thermal Physics Department of Physics, University of Surrey Problem Set 4: Heat Pumps and Engines You should read Chapter 4 of Thermal Physics by C.B.P. Finn, if you have not already. (1) Below is a P-V diagram for an ideal Stirling cycle. The process going from 1 to 2 is an isothermal expansion at a temperature of TH, and the process going from 3 to 4 is an isothermal compression at a temperature of TC, where TH > TC. The working substance of the Stirling engine based on this cycle can be approximated as an ideal gas. P 1 1 Q1 QA 2 4 QB Q2 3 V (i) (ii) If the heat into the system in going from 4 to 1 is equal to the heat being expelled when going from 2 to 3 (e.g. if the heat is “recycled”), show that the efficiency of the engine is given as (Q1 – Q2)/Q1. Then use the First Law of Thermodynamics to show that the expression for the efficiency of this Stirling engine is equal to (TH – TC)/TH. Thus, you can show that its efficiency is the same as a Carnot engine operating between the same two reservoirs. (2) The figure on the next page shows the cycle used for a hypothetical heat engine that uses an ideal gas as its working substance. Show that the efficiency of the engine, , is: (1 P3 / P1 ) 1 (1 V1 / V3 ) For discussion during Tutorial 4 in Week 8 on 23 November, 2011 2TP - Thermal Physics Department of Physics, University of Surrey P Q1 1 2 P1 Q2 Adiabatic P3 3 V1 V3 V (3) 3 kg of water at a temperature of 0 ºC are in a refrigerator. The room that contains the refrigerator is maintained at a constant temperature of 21 ºC. What is the minimum amount of work input into the refrigerator that is required to freeze the water? The latent heat of fusion for water is 3.33 x 105 J kg-1. (4) You wish to heat your house using a heat pump operating between the house and the outside. On a typical winter's night, the temperature outside is -5 ºC, and you wish the temperature inside the house to be at 23 ºC. You estimate that the rate of heat loss from the house is 20 kW. What is the minimum power required to operate the heat pump? (5) A satellite in outer space carries a Carnot engine that delivers a fixed amount of power at a rate of W . The temperature of the heat source is fixed at T1. The engine gives off heat to a cold reservoir at a temperature of T2. The cold reservoir consists of a large panel that dissipates heat by thermal radiation. The rate of heat dissipation matches the rate of heat delivery, so that temperature of the reservoir remains constant. The Stefan-Boltzmann equation states that the rate of thermal radiation is related to the absolute temperature of a body, T, as: AT4, where A is the surface area of the body and is the Stefan-Boltzmann constant. The size of the panel should be as small as possible, so that the satellite's weight is minimised. Show that the area of the panel has a minimum value when T2 has a value of 3T1/4. Hint: Derive a relationship for A as a function of T1 and T2. Then find when the function has a minimum value. For discussion during Tutorial 4 in Week 8 on 23 November, 2011 2TP - Thermal Physics Department of Physics, University of Surrey (6) Show that for the Carnot cycle shown in the diagram below that the volume ratios in the two isothermal expansions are the same. That is, show that Vb/Va = Vc/Vd. Hint: consider what you know about the volume relationship during the two adiabatic processes. This final question provides experience in making approximations when doing a calculation. Make appropriate estimates and approximations when solving it. (7) A closed, well-insulated room has dimensions of 10 m x 5 m x 3 m. The room temperature is 20 C. If a 10-kg copper block at a temperature of 150 C is placed in the room, what will be the final temperature of the room and block when thermal equilibrium is reached? For discussion during Tutorial 4 in Week 8 on 23 November, 2011