Activity 1
advertisement

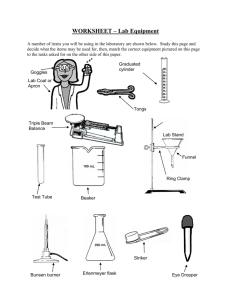
Perhaps the birds passed the flu to human. Scientists tried to find out if the patients and the birds got the same virus. Why did many people get flu after contact with birds? Observation Making hypothesis Both birds and patients got the same virus. Recording data and analysing Activity 1(e) Carrying out experiment Bird flu was passed from birds to humans. Drawing conclusion QUICK PRACTICE 1.2 The following are steps in a scientific investigation. Arrange them in the correct order. (a) Carry out experiments (b) Record and analyse (c) Make observations (d) Make a hypothesis (e) Draw a conclusion (c) (d) (a) (b) (e) Activity 1(e) Activity 1 (f) Laboratory 1. How many laboratories are there in your school? There are four. 2. What are their names? (You can check them out at their entrances.) They are Physics Lab., Biology Lab., Chemistry Lab. and Integrated Science Lab. 3. Where is your science laboratory(located on which floor)? It is on the 4th floor. Activity 1(e) 4. Write down three main differences between a science laboratory and a normal classroom. Science laboratory: Normal classroom: smaller bigger no laboratory with laboratory equipment equipment e.g. gas tap, sink with benches no benches Activity 1(e) Activity 1 (h) Functions of apparatus Look at the photos in Fig.1.9 carefully. Try to guess the functions of the following apparatus. Apparatus Function dropper to transfer a small amount of liquid reagent bottle conical flask, beaker to store solutions/liquids Activity 1(e) to hold solutions/liquids Apparatus Function spatula to transfer a small amount of powder glass rod to stir liquids Bunsen burner to heat things heat-proof mat to protect the bench safety spectacles to protect your eyes tongs Activity 1(e) to pick up and hold hot objects Apparatus Function measuring cylinder to measure the volume of liquids gas jar to hold gases test tube to hold a small amount of liquid test tube holder to hold a heating test tube rack to hold test tubes Activity 1(e) test tube during Put a red circle around each potential danger. Discuss why it is dangerous and suggest necessary precautions in each case. A C B G E H F D Activity 1(e) I A-I should not enter the laboratory unless the teacher is present. A should tie up her long hair and turn off the gas tap. B should not use a thermometer to stir the liquid. C should not touch the switch with a wet hand. Activity 1(e) D should not hold the test tube with a bare hand when heating. She should clean up the bench and keep paper away from the Bunsen burner. E should not smell the chemical directly. He should fan the gas gently to his nose. F should not throw solid waste into the sink. Activity 1(e) G should not point the mouth of a test tube with hot liquid towards another student. H should not pour solutions into the beaker from a high position, and he should wear safety goggles. I should clean up the bench and report the breakage to the teacher immediately. Also, she should not touch the wire gauze when it is hot. Activity 1(e) QUICK PRACTICE 1.3 Apparatus A B C E D G Name A reagent bottle B beaker C glass rod F D tripod E heat-proof mat F dropper G wire gauze Function to support wire gauze to protect the bench to hold liquids to store solutions to transfer a small amount of liquid to protect the beaker in heating to stir liquids Activity 1(e) XPERIMENT 1.1 Using a Bunsen burner Materials and apparatus Bunsen burner 1 matches 1 box heat-proof mat 1 safety spectacles 1 test tube 1 test tube holder 1 Activity 1(e) 2.(a) Observe the flame of the Bunsen burner. What is the colour and shape of the flame? The flame is yellowin colour and irregular (regular / irregular) in shape. The Bunsen flame you now observe is called the yellow flame (luminous flame or quiet flame). Activity 1(e) Draw the flame in the diagram on the right and colour it with a coloured pencil. yellow the air hole is closed Activity 1(e) 2.(b) Open the air hole slowly. The colour of the flame changes from yellow to blue . The shape of the flame becomes regular . The Bunsen burner becomes noisier (quieter / noisier). Activity 1(e) blue the air hole is open Activity 1(e) XPERIMENT 1.2 Demonstration Investigating the Bunsen flame Materials and apparatus tongs 1 Bunsen burner 1 glass tube 1 heat-proof mat 1 splint 1 wire gauze 1 pin 1 matches 1 box Activity 1(e) (b) Turn on the gas tap and light the Bunsen burner. What happens to the match? It does not light up. This shows that the inner cone of the Bunsen flame is not very hot (very hot / not very hot). inner cone match pin chimney Activity 1(e) 2. (a) Light the Bunsen burner. Open the air hole to produce a blue flame. (b) Hold a splint with a pair of tongs. Put it into the Bunsen flame about 0.5 cm above the chimney for two seconds. (If the splint burns, you can try again and shorten the time to one second.) splint 0.5 cm chimney Activity 1(e) tongs 2. (c) Remove the splint from the Bunsen flame and observe it carefully. Draw what you see. What does this tell us about the hotness of the inner cone? The temperature of the inner cone is not very high. Activity 1(e) (b)Now we want to find out which part of the Bunsen flame is the hottest. Put the wire gauze at different positions. wire gauze C A B At which position does the wire gauze glow first? It is B. Activity 1(e) 3. (c) Mark the hottest part of the Bunsen flame on the diagram with an ‘X’. X Activity 1(e) 4.(a) Hold a glass tube with a pair of tongs. Put one end of the tube into the inner cone of the Bunsen flame. (b) Light the other end with a burning splint. Does it burn? Yes, it does This shows that the inner cone contains unburnt (burnt / unburnt) gas. Activity 1(e) XPERIMENT 1.3 Using a dropper to transfer solution Materials and apparatus test tubes 2 dropper 1 test tube rack 1 coloured liquid Activity 1(e) What will happen if you squeeze the rubber bulb too much? The coloured liquid will be sucked into the rubber bulb . What will happen if you hold the dropper upside down? Should we use the dropper like this? Why? The liquid will go into the rubber bulb and it may be contaminated by other chemicals left behind in the bulb. Activity 1(e) XPERIMENT 1.4 Mixing solution Materials and apparatus test tubes 9 beaker (100cm3) test tube rack 1 different solutions dropper 1 Activity 1(e) 1 1. Your teacher will give you eight bottles of solutions labelled A to H. Note the colour of each solution. Write down your observations in the table below. Solution Colour Solution Colour A B C D colourless E F G H clear yellow Activity 1(e) colourless clear blue colourless colourless colourless clear green (c) Mix well by shaking the tube. Caution If you spill any solution on your hands, wash them immediately in running water. Observe carefully what happens. Write down your observations. The colour of the mixed solution is yellow . Activity 1(e) Colour of Solutions Is it clear Any solids the mixed or cloudy? formed? solution Any bubbles formed? yellow clear brown cloudy white / clear light blue blue cloudy orange clear white clear blue cloudy no no no no no no no A+B B+C A+C C+D E+G A+H C+F Activity 1(e) yes yes yes yes no yes yes 4. You can also mix the solutions by pouring them directly out of the bottles into the test tubes. Compared with this, what are the advantages of using a dropper? It is easier to control the amount of solution transferred, and the solution is less likely to spill. Caution Wash your hands after the experiment. Activity 1(e) QUICK PRACTICE 1.4 1. The following are the steps for lighting a Bunsen burner. Arrange them in the correct order. (a) Make sure that the rubber tubing is properly connected to the gas tap. (b) Light a match. (c) Close the air hole. (d) Hold the burning match just above the top of the chimney and turn on the gas tap. (e) Put the Bunsen burner on a heat-proof mat. (e) or (c) (a) Activity 1(e) (c) (e) (b) (b) (d) (d) Activity 1 (j) Choosing the unit for measuring lengths What units would you use to represent the length of the following things? mm Activity 1(e) cm m Activity 1 (k) Correct way of measuring lengths Three students are measuring the length of a pencil. Who is in the proper position to read the scale? It is B. 7.5 cm 7 cm 8 cm B A Activity 1(e) C XPERIMENT 1.6 Using a thermometer to measure temperatures Materials and apparatus thermometer 1 ice beaker (250 cm3) 1 hot water Activity 1(e) 1. Measure the room temperature with a thermometer. The room temperature is Caution ºC. Mercury is poisonous. When you are using a mercury thermometer, extra care needs to be taken. If you accidentally break a thermometer, do not touch the mercury or try to pick up the pieces of broken glass. Inform your teacher immediately. Activity 1(e) 2. What is the temperature of tap water? Caution Do not stir water with a thermometer. You may break the thermometer. Activity 1(e) 3. Your teacher will give you some hot water. What is the temperature of the hot water? Activity 1(e) 4. Your teacher will give you some ice water. What is the temperature of the ice water? Activity 1(e) 5. Hold the bulb of the thermometer in your hand for one minute. What temperature does it show? Activity 1(e) XPERIMENT 1.9 Using a measuring cylinder to measure volumes Materials and apparatus measuring cylinder (100 cm3) 1 empty cans of similar containers several displacement can 1 plasticine block 1 stone 1 thread Activity 1(e) (b) The correct reading is taken from the bottom of the meniscus. Also, make sure that your eyes are at the same level as the bottom of the meniscus. (c) The volume of tap water in the above measuring cylinder is 79 cm3. Activity 1(e) Type of container Labelled volume (cm3) Measured volume (cm3) Is the measured volume equal to the labelled volume? If not, can you explain why? Activity 1(e) II.Measuring the volumes of irregular solids 1 (a) Pour tap water into a measuring cylinder until it is about half full. The volume of water in the measuring cylinder is cm3. Activity 1(e) The new reading is cm3. The volume of the plasticine block is cm3. The volume of a solid is equal to the volume of water it displaces. If the plasticine block is divided into two smaller pieces, do you think its volume will change? Activity 1(e) The reading is cm3. The volume of the plasticine blocks is cm3. Does the volume of the plasticine block change? No, it doesn’t What can you conclude after this step? The volume of a solid does not change even when it is divided into many pieces. Activity 1(e) 3. Some irregular-shaped objects are too large to fit into a measuring cylinder. Their volumes can be measured by a displacement can. (a)Tie a large stone with a thread. (b) Fill the displacement can with water until a small amount runs out of the spout. Activity 1(e) (d) Slowly lower the stone into the displacement can. Measure the volume of water that flows out of the can into the measuring cylinder. displacement Volume of the stone can = volume of water spout collected by the measuring measuring cylinder cylinder 3 = cm . stone Activity 1(e) QUICK PRACTICE 1.5 1. Complete the table below. Instrument Activity 1(e) Name of the What does it Units of instrument measure? measurement stop-watch time second measuring cylinder volume cm3 QUICK PRACTICE 1.5 1. Complete the table below. Instrument Name of the instrument What does it Units of measure? measurement thermometer temperature balance Activity 1(e) weight oC kg QUICK PRACTICE 1.5 2. State the instrument you will use to measure the following. Also, state the unit in each case. Measurement Instrument Unit Temperature of tap water Time required to boil a beaker of water The weight of a 10-cent coin Activity 1(e) thermometer oC stop-watch s/min electronic balance/ balance g Activity 1 (o) Are the experiments fair? Three students carry out an experiment to find out which brand of battery, A or B, has a longer life. They use the batteries to power torches until the batteries run out. Brand A battery Activity 1(e) Brand B battery Anthony’s experiment Leo’s experiment new batteries Eric’s experiment Activity 1(e) old batteries Are the student’s experiment fair? Anthony’s experiment is unfair (fair / unfair) because the bulbs of the two torches are . different Leo’s experiment is unfair (fair / unfair) because new and old batteries are used . Eric’s experiment is fair (fair / unfair) because all the batteries are new and the bulbs are the same Activity 1(e) . Activity 1 (p) Goose down jacket and silk jacket A goose down jacket is better at keeping us warm. Activity 1(e) No! A silk jacket is better. In winter, we often wear a goose down jacket or silk jacket. Do you know which of them is better at keeping us warm? Let us design a fair test to find out. 1. What is your hypothesis? Goose down jacket is better at keeping us warm because it prevents heat loss better than a silk jacket. Activity 1(e) 3. How are you going to carry out the experiment? Draw a diagram to show your experimental set-up. same volume of water goose down jacket Activity 1(e) silk jacket 4. What measurements will you make? I will measure the changes in the temperature of water in the bottles. 5. How can you make sure that your test is fair? I will fill the bottles with the same (the same / different) amount of hot water. I will measure the changes in water temperature at the same (the same / different) time. Activity 1(e) 6. How will you draw your conclusion? The jacket that covers the bottle with a smaller drop in water temperature is better (better / poorer) at keeping us warm. Activity 1(e) QUICK PRACTICE 1.6 1. Draw a vertical section diagram of each of the following: a watch glass on a conical flask a test tube beaker Activity 1(e) QUICK PRACTICE 1.6 2. Samuel carries out a fair test as shown below to compare the heat energy produced by a yellow flame and a blue flame. thermometers water yellow flame Activity 1(e) blue flame QUICK PRACTICE 1.6 Complete the variable table below. Controlled Independent Dependent variables variables variables • amount of water • yellow flame or • the rise in water • temperature of blue flame temperature water at the beginning • time of heating the water • size of the beakers • size of the flame Activity 1(e)