216814_lab_reaction_12
advertisement

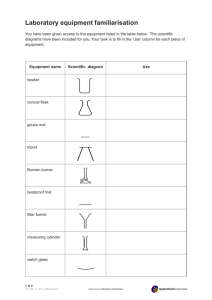
Lab: Introductory Chemical Reactions Purpose: The purpose of the following experiments is to observe several chemical reactions, classify them as one of the five reaction types, and write balanced equation for them. When doing each experiment record the following: Materials: Procedure: Data (a description of the reaction – be sure to look for details that will allow you to determine the products of the reaction) Balanced equation Reaction 1: Synthesis 1. Obtain 10 mL of a O.10 M CuSO4 solution. Place in a test tube. 2. Place 0.32 g granular copper metal and 0.16 grams powdered sulfur in a weigh boat and mix together thoroughly with a spatula. 3. Heat the test tube very gently by bringing it slowly in and out of the Bunsen burner flame until it just starts to boil. CAUTION: Do not allow to boil or solution can “fly” out of test tube. ALWAYS: point test tube away from other students. 4. Stir the Cu/S mixture into the boiling solution. Wash any extra into the solution. 5. Continue to heat near boiling until black solid forms. 6. Set up filtration and collect the black solid on a piece of filter paper. Record observations. Reaction 2: Decomposition. Place a small portion of copper (II) carbonate in a clean and dry minitube. Heat gently. Record your observations. When writing the balanced equation, note that carbon dioxide is one of the products. Reaction 3: Double Replacement 1. Take the reaction product from reaction 2 over to the hood. (Be ready to say what the reaction product is!) 2. With the teacher’s assistance, add a few drops of 12M HCl. Record observations and figure out the balanced equation Reaction 4: Single Replacement 1. Place 10.0 mL of 0.10 M CuSO4 solution in a test tube. 2. Add a nail (iron). 3. For a control, add a nail to a test tube containing DI water. 4. Make detailed observations. Reaction 5: Combustion 1. Light your Bunsen burner. 2. Using beaker tongs, move a beaker with ice in and out of the flame at a height of about 10 cm. Notice the product from the reaction appear on the sides of the beaker. Record observations. 3. Next, light a wood splint. Hold the flame under the open end of the test tube for about 15 seconds. Extinguish the flame and turn the test tube upright. Squirt in one pipette full of limewater. Vortex and record any change to the limewater. 4. Record all observations. The reaction is the burning of methane, however the burning of the wood splint does produce the same two products, both of which you should have just determined.