The Second law
advertisement

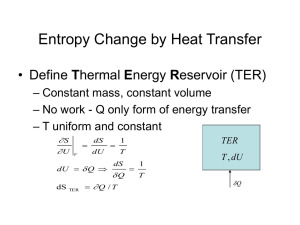
* Reading Assignments: 3.1 3.1.1 3.2 3.3 3.4 3.4.1 3.4.2 3.5 3.6 3.6.1 4. The Second Law 4.1 Reversible vs Irreversible processes Reversible: A process for which a system can be restored to its initial state, without leaving a net influence on the system or its environment. * idealized, frictionless; * proceeds slowly enough for the system to remain in thermodynamic equilibrium. Irreversible: Not reversible * natural; * proceeds freely, drives the system out of thermodynamic equilibrium; * interacts with environment, can not be exactly reversed Example: Gas-piston system under a constant temperature ( du 0 ) * Slow expansion and compression ( p p ps ) q w pdv w 12 w21 0 * Rapid expansion ( p p ps ) and compression ( p p ps ) q w pdv w 12 w21 0 4.2 Entropy / Carnot’s Theorem * Consider the first law, q cv dT pdv , or q c p dT vdp Divide them by T and use pv=RT, q dT dv cv R , T T v or q dT dp cp R T T p The specific entropy (s) is defined as, ds q T which is a state variable, a property of the system. * The Carnot cycle and Carnot Theorem 1) From state 1 to state 2: Isothermal expansion T2 T1 T12 , v2 v1 u12 0 q12 w12 2 1 v2 pdv RT12 ln v1 2) From state 2 to state 3: Adiabatic expansion T34 T3 T2 T12 , v3 v2 q23 0 w23 u23 cv (T34 T12 ) 3) From state 3 to state 4: Isothermal compression T4 T3 T34 , v4 v3 u34 0 v q34 w34 pdv RT34 ln 4 3 v3 4 4) From state 4 to state 1: Adiabatic compression T12 T1 T4 T34 , v1 v4 q41 0 w41 u41 cv (T12 T34 ) The net heat transfer and the net work over the Carnot cycle are: v4 v2 w q RT12 ln v1 RT34 ln v3 Using Poisson’s equation, v3 T12 T34 v2 1 v4 v1 1 , v2 v3 v1 v4 Then, v2 0 v1 w q R(T12 T34 ) ln So the system absorbs heat and performs net work in the Carnot cycle, which behaves as a heat engine. Why is the Carnot cycle reversible? For the Carnot cycle, we can also have: v2 v4 q12 q34 R ln ln 0 T T12 T34 v3 v1 q This relationship also hold for the reversed Carnot cycle. This is called the Carnot’s Theorem: ds 0 , q ds T rev (4.1) which shows that the change of entropy is independent of path under a reversible process. 4.3 The Second Law and its Various Forms To get the second law, we use the Clausius Inequality, i.e., for a cyclic process, q T 0 which indicates during the cycle, 1) heat must be rejected to the environment somewhere during a cycle; 2) heat exchange is larger at high temperature than at low temperature under reversible conditions; 3) the net heat absorbed is smaller under the irreversible condition than under the reversible condition. Now, consider two cycles as shown in the plot. For the cycle which contains one reversible process and one irreversible process, 2 1 q T rev q 2 T irrev 0 1 (4.2) For the cycle which have two reversible processes, 2 1 q T rev q 2 T rev 0 1 (4.3) The difference between (4.2) and (4.3) gives q 2 T rev 1 1 2 ds q 2 T irrev 1 Because states 1 and 2 are arbitrary, we have the second law, ds q (4.4) T Combine (4.1) and (4.4), we have q q T T rev It indicates that the heat absorbed by the system during a process has an upper limit, which is the heat absorbed during a reversible process. The first law relates the state of a system to work it performs and heat it absorbs. The second law controls how the systems move to the thermodynamic equilibriums, i.e., the direction of processes. Several simplified forms of the second law: 1) For an adiabatic process, (4.4) becomes ds 0 (4.5) If the adiabatic process is reversible, then ds 0 It is also isentropic (s is constant). 2) For an isochoric process, (4.1) becomes dT dT dsv cv cv T rev T v (4.6) Because only state variables are involved, it holds for either reversible or irreversible processes. (4.5) and (4.6) show that : Irreversible work can only increase entropy; heat transfer can either increase or decrease entropy. 4.4 Fundamental Relations / The Maxwell Relations Combine forms of the first law and the second law, ds T cv dT dv p T T Tds du pdv or Similarly, q du Tds pdv (4.7) q dT dp ds cp v T T T Tds dh vdp or dh Tds vdp (4.8) For reversible processes, the equal signs apply and the equations are called Fundamental Relations, du Tds pdv (4.9) dh Tds vdp (4.10) Because these equations involve only state variables, they do not depend on path. So they must hold for both reversible and irreversible processes. These identities describe the change in one state variable in terms of changes in two other state variables. Two other state variables can be defined, f u Ts The Helmholtz function: The Gibbs function: g h Ts u pv Ts Use these definitions in (4.7)-(4.10), df sdT pdv (4.11) dg sdT vdp (4.12) df sdT pdv (4.13) dg sdT vdp (4.14) * The Maxwell Relations: Recall that the condition for a function to be exact is, dz Mdx Ndy M N y x Since the state variables are exact, so for u, from du Tds pdv We have T p v s s v (4.15) Similarly from the fundamental relations we can show that s p v T T v (4.16) T v p s s p (4.17) s v T p p T (4.18) (4.15)-(4.18) are called the Maxwell relations. * Noncompensated Heat Transfer For an irreversible process, du Tds pdv To remove the inequality, a term can be added to the right side of the formulation, du Tds pdv q Next, use the second law for a reversible process on du, Trev ds prev dv Tds pdv q Finally, q (T Trev ) ds ( p prev ) dv q is called the noncompensated heat transfer, which measures additional heat rejection to the environment due to irreversibility. 4.5 Thermodynamic Equilibrium * Consider an adiabatic process, the second law becomes ds 0 For an irreversible condition, ds 0 or s0 s s0 is the entropy at the initial state. When s reaches the maximum, the state is in thermodynamic equilibrium because the entropy can not increase anymore. * Consider an isentropic-isochoric process, From (4.7), we have du 0 For an irreversible condition, du 0 or u0 u u0 is the internal energy at the initial state. When u reaches the minimum, the state is in thermodynamic equilibrium because the internal energy can not decrease any further. * The enthalpy, Helmholtz function and Gibbs function must all decrease as a system reaches thermodynamic equilibrium under certain processes. 4.6 Relationship of Entropy to Potential Temperature * Use the first law and the equation of state for an ideal gas in the second law, ds q T cp dT dp R T p ds R d ln T d ln p cp cp * use log derivatives on the potential temperature, R cp p ln ln T 0 p R d ln d ln T d ln p cp So, ds d ln cp (4.19) Under the reversible process, we have ds d ln cp (4.20) So, the change in entropy can be measured by the change in potential temperature. Because (4.20) involves only state variables, it is path independent and is valid for both reversible and irreversible processes. 4.7 Implications for Vertical Motion 1) Under adiabatic processes: d 0 , and ds 0 Adiabatic conditions require: a. no heat be transferred between the system and environment; b. no heat exchange between one part of the system and another. So, these exclude the irreversible turbulent mixing and the irreversible expansion work-induced mixing in the system. Therefore, the adiabatic process is approximately reversible, i.e., d 0 , and ds 0 So, the potential temperature surfaces ( const ) coincide with Isentropic surfaces ( s const ) . An air parcel will remain on a certain Isentropic surface and undergoes no systematic vertical motion. 2) Under diabatic processes: ds q d ln cp c pT An air parcel moves across isentropic surfaces following the heat transfer with its environment. 3) The displaced motion is sufficiently slow: The air parcel’s temperature differs from the environment only infinitesimally; Rejection of heat during the poleward moving is balanced by the absorption of heat during the equatorward moving. No net vertical motion, the parcel’s evolution is reversible. 4) The displaced motion is sufficiently fast: Produce the net heat transfer and a vertical drift of the parcel across isentropic surface in a complete cycle. Meteorology 341 Homework (3) 1. A dry air parcel undergoes a complete Carnot cycle consisting of the steps indicated in (a)-(d). For each individual step, calculate the mechanical work w (per unit mass) done by the air parcel and the heat q added to the parcel. (a) Adiabatic compression from p1=600 hPa and T1=0oC to a temperature T2 of 25oC; (b) Isothermal expansion to a pressure p3 of 700 hPa; (c) Adiabatic expansion to temperature T4 of 0oC; (d) Isothermal compression back to the original pressure p1. Also, compute (e) the total work done and heat added for the complete cycle, and (f) the efficiency of the cycle. 2. Two hundred grams of mercury at 100oC is added to 100 g of water at 20oC. If the specific heat capacities of water and mercury are 4.18 and 0.14 JK-1g-1, respectively, determine (a) the limiting temperature of the mixture, (b) the change of entropy for the mercury, (c) the change of entropy for the water, and (d) the change of entropy for the system as a whole. 3. During a cloud-free evening, LW heat transfer with the surface causes an air parcel to descend from 900 to 910 mb and its entropy to decrease by 15 J kg-1 K-1. If its initial temperature is 280 K, determine the parcel’s (a) final temperature and (b) final potential temperature.