eprint_12_29705_298
advertisement

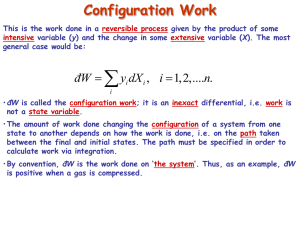
2 the expansion of the gas :for ideal gas PV=nRT R=gas constant =8.314 J/k .mole=0.08206 Mole:amount of substance of a system which contains as many elementry entities (atoms,moleclues,ions) Q and w not thermodynamic variables. Intensive properties :they are do not depend on the mass of the system such as viscocity, density n, surface tension. Extensive prop[erties : they are depend on the amount of substance or substances present in the system.,such as internal energy, entropy ,volume Thermodynamics reveresible process :in this process the gas can be returned to its original state without any change in either the system or its surroundings and the system at all process remain in equilibrium with its surrounding and the process is infiitly slow. The heat content the enthalpy=(ub-ua)+p(vb-va) There is isothermal reversible process where dT=0, T =CONSTANT THE ADIABATIC REVERSIBLE PROCESS WHERE Q=0 PAVA=PBVB The heat content the enthalpy=(ub-ua)+p(vb-va) There is isothermal reversible process where dT=0, T =CONSTANT THE ADIABATIC REVERSIBLE PROCESS WHERE Q=0 PAVA=PBVB FOR ANIDEAL GAS CP-CV=R FOR ANIDEAL GAS CP-CV=R