Ch20
advertisement
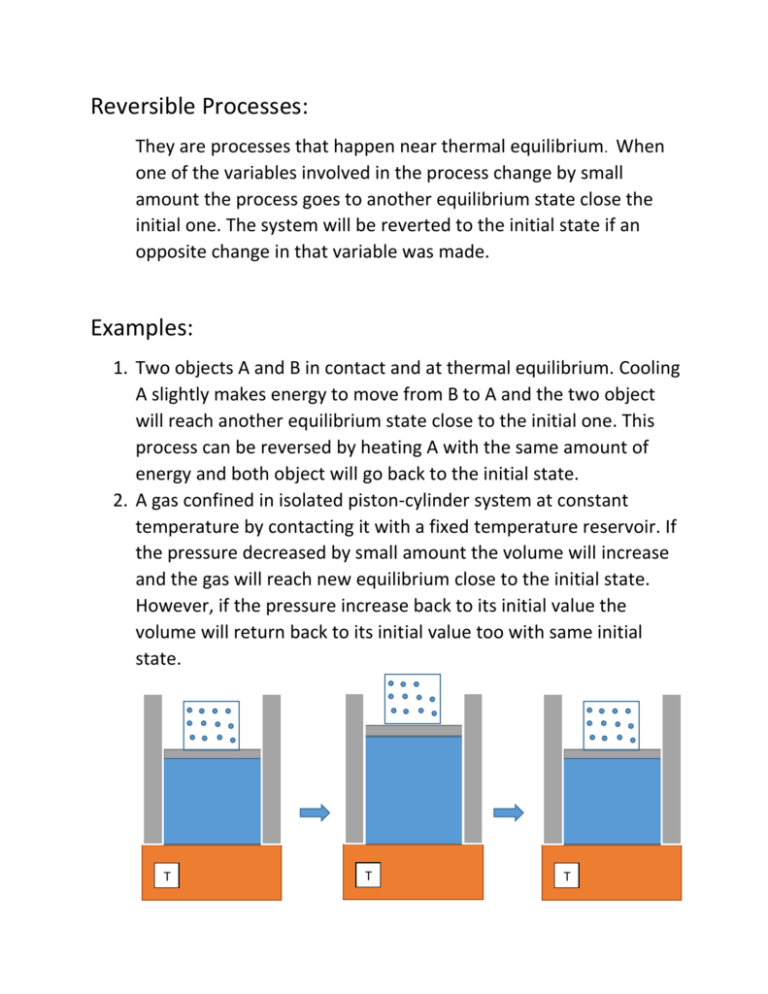
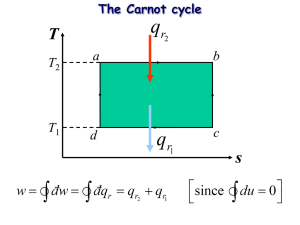
Reversible Processes: They are processes that happen near thermal equilibrium. When one of the variables involved in the process change by small amount the process goes to another equilibrium state close the initial one. The system will be reverted to the initial state if an opposite change in that variable was made. Examples: 1. Two objects A and B in contact and at thermal equilibrium. Cooling A slightly makes energy to move from B to A and the two object will reach another equilibrium state close to the initial one. This process can be reversed by heating A with the same amount of energy and both object will go back to the initial state. 2. A gas confined in isolated piston-cylinder system at constant temperature by contacting it with a fixed temperature reservoir. If the pressure decreased by small amount the volume will increase and the gas will reach new equilibrium close to the initial state. However, if the pressure increase back to its initial value the volume will return back to its initial value too with same initial state. T T T 3. Mixing similar material samples at the same state. 4. Frictionless motion. Irreversible Processes: The process is irreversible, if the system cannot return back to its initial state when the process is reversed. The system are not at equilibrium throughout the process. Examples: 1. When two objects A and B at high temperature difference are but in contact with each other. We cannot reverse the change in their temperatures by small energy transfers. 2. TA ≠ TB A B A B TA TB Tf Tf 3. Large sudden gas pressure change. i Irreversible process f Compare to i Reversible process f T 4. Mixing of fluids of different type or at different temperature. 5. Objects at different temperature connected through another material. A B TA TB 6. Motion with friction. 7. Free expansion. ( Q = 0 & W = 0 V1 ΔEint = 0 T = constant) V2 Initial state i Vi = V1 Final state f Vf = V1 + V2 Compare to reversible isothermal process ( ΔEint = 0 → dQ = dW = PdV) i f T T V = V1 𝑓 𝑑𝑄 ∆𝑆 = ∫𝑖 𝑇 = V = V1 + V2 1 𝑓 1 𝑓 1 𝑓 ∫ 𝑑𝑄 = 𝑇 ∫𝑖 𝑃𝑑𝑉 = 𝑇 ∫𝑖 𝑛𝑅𝑇 𝑇 𝑖 𝑑𝑉 𝑉 = 𝑛𝑅 𝑙𝑛 𝑉𝑓 𝑉𝑖 Entropy: It is a state function that reflect microscopically the degree of disorder. Macroscopically for reversible process its change can be calculated by the following formula: 𝑓 ∆𝑆𝑟𝑒𝑣 = ∫ 𝑖 𝑑𝑄𝑟𝑒𝑣 𝑇 Work done by irreversible process is always less than work done in reversible process that have the same initial and final states. Entropy is extensive thermodynamic variable. The value of entropy of complex system is the sum of the entropy of its constituents. Since entropy is a state function, then its change depends on the initial and final states only, not on the path (process) that take the gas from the initial state to final state.