Thermodynamics Practice Problems & Solutions
advertisement
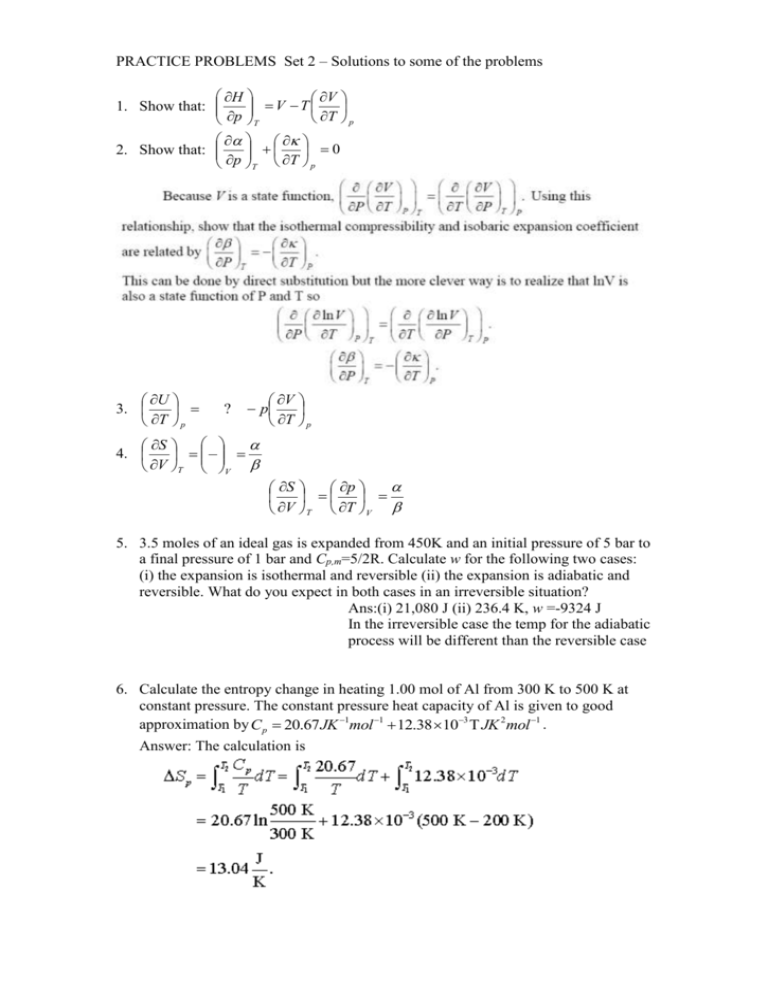
PRACTICE PROBLEMS Set 2 – Solutions to some of the problems H V V T 1. Show that: T p p T 2. Show that: 0 p T T p U 3. T p ? V p T p S 4. V T V p S V T T V 5. 3.5 moles of an ideal gas is expanded from 450K and an initial pressure of 5 bar to a final pressure of 1 bar and Cp,m=5/2R. Calculate w for the following two cases: (i) the expansion is isothermal and reversible (ii) the expansion is adiabatic and reversible. What do you expect in both cases in an irreversible situation? Ans:(i) 21,080 J (ii) 236.4 K, w =-9324 J In the irreversible case the temp for the adiabatic process will be different than the reversible case 6. Calculate the entropy change in heating 1.00 mol of Al from 300 K to 500 K at constant pressure. The constant pressure heat capacity of Al is given to good approximation by C p 20.67 JK 1mol 1 12.38 103 JK 2 mol 1 . Answer: The calculation is 7. 10 moles of a gas are contained under high pressure so that the volume is 0.5 L. The external pressure is suddenly reduced and the gas allowed to expand into a 50L container. Assume the gas is a van der Waals gas with a=3.7 atm L2 mol-2 and b=0.043 Lmol-1. The temperature is constant at 298K. Determine the change in entropy of the gas for this volume change. 8. The composition of dry air is approximately 78% N2, 21% O2, and 1% Ar by volume (which is the same as mole percent). What is the molar entropy of mixing of air? 9. Determine the value of G per mole for the freezing of supercooled water at -60C. (Latent heat of fusion of ice 80 cal gm-1) 10. Find the change in Gibbs free energy accompanying the compression of 1 mole of ethane at 500C from 20 to 250 bars. Hint: Use pressure dependence formula











