Entropy Change & Heat Transfer: Thermodynamics Presentation
advertisement
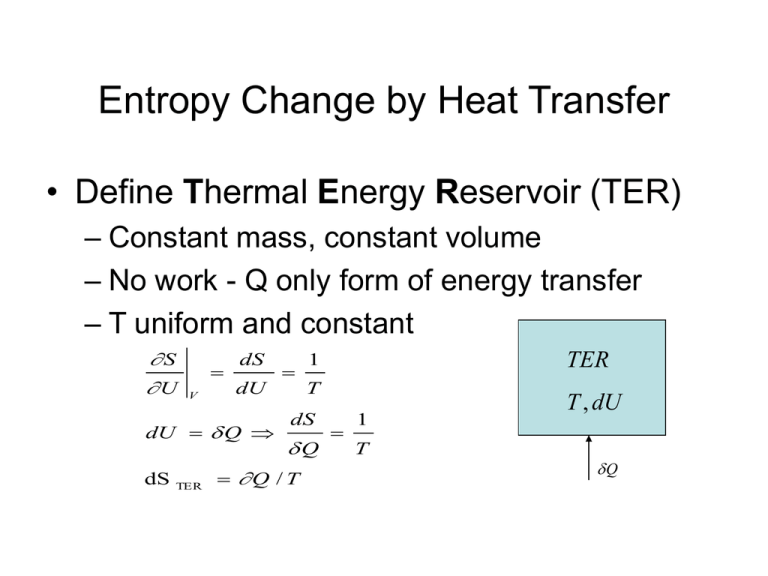
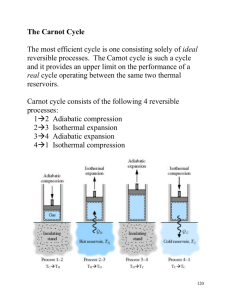
Entropy Change by Heat Transfer • Define Thermal Energy Reservoir (TER) – Constant mass, constant volume – No work - Q only form of energy transfer – T uniform and constant S U V dS dU dU Q dS TER TER 1 T dS Q T , dU 1 T Q Q / T Entropy Change by Heat Transfer • Consider two TERs at different Ts, in contact but isolated from surroundings PS d ( S A S B ) Q ( 1 TA Heat transfer between TERs produces entropy as long as TB>TA 1 TB )0 TER TA Q TER TB Second Law for Control Mass • Mechanical Energy Reservoir (MER) • CM interacts with a TER and an MER • MER no disorder; MER W provides only reversible work CM , T Q • Overall system isolated rev TER 2nd Law PS dS system PS d ( S TER S MER S CM ) PS Q T dS CM dS CM Q T PS 0 No entropy change could occur because: - Isentropic process (Ps = 0) - entropy production cancelled by heat loss Ps - Q/T = 0 Alternative Approach to 2nd Law Clausius • It is impossible to design a cyclic device that raises heat from a lower T to a higher T without affecting its surroundings. (need work) Kelvin-Planck • It is impossible to design a cyclic device that takes heat from a reservoir and converts it to work only (must have waste heat) Carnot’s Propositions • Corollaries of Clausius and KelvinPlanck versions of 2nd Law: 1. It is impossible to construct a heat engine that operates between two TERs that has higher thermal efficiency than a reversible heat engine. th,rev> th,irrev 2. Reversible engines operating between the same TERs have the same th,rev Carnot (Ideal) Cycle • Internally reversible • Interaction with environment reversible Qh T Win Wout Reversible work S - constant Reversible heat transfer T - constant QL S Carnot efficiency • Define efficiency: W th PS net , out QH QH TH Q H QL QH TH Carnot Ql TL TH QL QH Ql QH TL over a reversible cycle QH 1 QH QL efficiency th ,Carnot 1 PS 0 TH W TL QL : TL TH TL This is the best one can do Gibbs Equation • State equations relate changes in T.D. variables to each other: e.g., q - w = du • If reversible and pdv work only Tds = du + pdv • In terms of enthalpy: dh = du + d(pv) dh = du + pdv + vdp; Tds = dh -vdp-pdv+pdv Tds = dh - vdp Unique aspect of Thermodynamics • The Gibbs Equations were derived assuming a reversible process. • However, it consists of state variables only; i.e., changes are path independent. • Proven for reversible processes but applicable to irreversible processes also. Enthalpy Relations for a Perfect Gas du Gibbs : ds = Integrate s : 2 1 T 2 1 ds = cv p T dv 2 1 c v dT T c v dT R 2 1 v R dv dv v R ln 1 T 2 dT Show yourself: s 2 1 cp p R ln 1 T p 2 dT fn (T) fn (p) Calculating s • Calculate temperature and pressure effects separately 0 12 s 0 2 0 1 s s where s 0 T T0 cp dT T sO(T) values are tabulated for different gases in Tables D For a Calorically Perfect Gas T p T s12 c p ln 2 R ln 2 c v ln 2 R ln 2 T1 p1 T1 1 T p T p ln 2 ln 2 ln 2 ln 2 R R T1 p1 1 T1 p1 s12 cp T 1 p ln 2 ln 2 R T1 p1 s12 T2 T1 1 ln p 2 p1