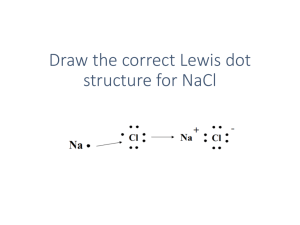
Acids and Bases: Brønsted-Lowry & Lewis Definitions
advertisement

Acids and Bases Brønsted-Lowry Acids and Bases • A Brønsted-Lowry acid is a proton donor. • A Brønsted-Lowry base is a proton acceptor. • H+ = proton Figure 2.1 Examples of Brønsted–Lowry acids and bases 1 Some molecules contain both hydrogen atoms and lone pairs and thus, can act either as acids or bases, depending on the particular reaction. An example is the addictive pain reliever morphine. 2 Which of the following molecules is not a BromstedLowry acid? HBr NH3 CCl4 Bromsted-Lowry acid must contain a proton. Which of the following is not a Bromsted-Lowry base? H3C CH3 O Bromsted-Lowry bases must have a lone pair or pi bond to donate. 3 Reactions of Brønsted-Lowry Acids and Bases • A Brønsted-Lowry acid base reaction results in the transfer of a proton from an acid to a base. • In an acid-base reaction, one bond is broken, and another one is formed. • The electron pair of the base B: forms a new bond to the proton of the acid. • The acid H—A loses a proton, leaving the electron pair in the H—A bond on A. 4 • The movement of electrons in reactions can be illustrated using curved arrow notation. Because two electron pairs are involved in this reaction, two curved arrows are needed. • Loss of a proton from an acid forms its conjugate base. • Gain of a proton by a base forms its conjugate acid. • A double reaction arrow is used between starting materials and products to indicate that the reaction can proceed in the forward and reverse directions. These are equilibrium arrows. Examples: 5 Label the acid, base, conjugate acid and conjugate base in the following reaction. O O + O CH3 + HO H3C H3C CH3 CH2 CA CB B A CH3 Use curved arrows to show the movement of electron pairs. O O + O CH3 + HO H3C CH2 H3C CH3 CH2 H 6 Draw the products of the following reaction ( use curved arrows to show movement of electron pairs) H3C H3C H H + NH2 NH2 HCl H + H H Cl + + H3C NH3 + Cl H H H + H H 7 Acid Strength and pKa • Acid strength is the tendency of an acid to donate a proton. • The more readily a compound donates a proton, the stronger an acid it is. • Acidity is measured by an equilibrium constant. • When a Brønsted-Lowry acid H—A is dissolved in water, an acid-base reaction occurs, and an equilibrium constant can be written for the reaction. 8 Because the concentration of the solvent H2O is essentially constant, the equation can be rearranged and a new equilibrium constant, called the acidity constant, Ka, can be defined. It is generally more convenient when describing acid strength to use “pKa” values than Ka values. 9 10 Factors that Determine Acid Strength • Anything that stabilizes a conjugate base A:¯ makes the starting acid H—A more acidic. • Four factors affect the acidity of H—A. These are: Element effects Inductive effects Resonance effects Hybridization effects • No matter which factor is discussed, the same procedure is always followed. To compare the acidity of any two acids: o Always draw the conjugate bases. o Determine which conjugate base is more stable. o The more stable the conjugate base, the more acidic the acid. 11 Element Effects—Trends in the Periodic Table. Across a row of the periodic table, the acidity of H—A increases as the electronegativity of A increases. Positive or negative charge is stabilized when it is spread over a larger volume. 12 • Down a column of the periodic table, the acidity of H—A increases as the size of A increases. • Size, and not electronegativity, determines acidity down a column. • The acidity of H—A increases both left-to-right across a row and down a column of the periodic table. • Although four factors determine the overall acidity of a particular hydrogen atom, element effects—the identity of A—is the single most important factor in determining the acidity of the H—A bond. 13 Factors that Determine Acid Strength—Inductive Effects • An inductive effect is the pull of electron density through bonds caused by electronegativity differences between atoms. • In the example below, when we compare the acidities of ethanol and 2,2,2-trifluoroethanol, we note that the latter is more acidic than the former. 14 • The reason for the increased acidity of 2,2,2trifluoroethanol is that the three electronegative fluorine atoms stabilize the negatively charged conjugate base. 15 • When electron density is pulled away from the negative charge through bonds by very electronegative atoms, it is referred to as an electron withdrawing inductive effect. • More electronegative atoms stabilize regions of high electron density by an electron withdrawing inductive effect. • The more electronegative the atom and the closer it is to the site of the negative charge, the greater the effect. • The acidity of H—A increases with the presence of electron withdrawing groups in A. 16 Factors that Determine Acid Strength—Resonance Effects • Resonance is a third factor that influences acidity. • In the example below, when we compare the acidities of ethanol and acetic acid, we note that the latter is more acidic than the former. • When the conjugate bases of the two species are compared, it is evident that the conjugate base of acetic acid enjoys resonance stabilization, whereas that of ethanol does not. 17 • Resonance delocalization makes CH3COO¯ more stable than CH3CH2O¯, so CH3COOH is a stronger acid than CH3CH2OH. • The acidity of H—A increases when the conjugate base A:¯ is resonance stabilized. 18 • Electrostatic potential plots of CH3CH2O¯ and CH3COO¯ below indicate that the negative charge is concentrated on a single O in CH3CH2O¯, but delocalized over both of the O atoms in CH3COO¯. 19 Factors that Determine Acid Strength—Hybridization Effects • The final factor affecting the acidity of H—A is the hybridization of A. Let us consider the relative acidities of three different compounds containing C—H bonds. • The higher the percent of s-character of the hybrid orbital, the closer the lone pair is held to the nucleus, and the more stable the conjugate base. 20 21 Figure 2.5 Summary of the factors that determine acidity 22 Calculate the pka’s and which is the stronger acid? OH ka= pka= CH3 10-10 10-41 10 41 OH 23 Which is the strongest base? H20 (pka= 15.7) NH3 (pka= 38) CH4 (pka= 58) CH4 Draw the products and determine the direction of the equilibrium. CH4 pka= 50 + OH CH3 + H2O 15.7 24 Which of these bases is strong enough to deprotonate CH3COOH (pka= 4.8) ? H Cl pka= 25 35 -7 Both acetylene and hydrogen will work (must have higher pka) Not using pka, which is the stronger acid? H2S HBr HBr, b/c Br is further to the right and further down in the periodic table. 25 Which is the stronger acid? F2CHCH2COOH OR Cl2CH2COOH F2CHCH2COOH is the most acidic b/c acids are stabilized by electron withdrawing groups and F is more electronegative than Cl Which is the stronger acid and why? H H H B/c the C-H bond involves sp2 hybridized orbital instead of sp3 26 Commonly Used Acids in Organic Chemistry In addition to the familiar acids HCl, H2SO4 and HNO3, a number of other acids are often used in organic reactions. Two examples are acetic acid and p-toluenesulfonic acid (TsOH). 27 Commonly Used Bases in Organic Chemistry Common strong bases used in organic reactions are more varied in structure. 28 It should be noted that: • Strong bases have weak conjugate acids with high pKa values, usually > 12. • Strong bases have a net negative charge, but not all negatively charged species are strong bases. For example, none of the halides F¯, Cl¯, Br¯, or I¯, is a strong base. • Carbanions, negatively charged carbon atoms, are especially strong bases. A common example is butyllithium. • Two other weaker organic bases are triethylamine and pyridine. 29 Lewis Acids and Bases • The Lewis definition of acids and bases is more general than the BrØnsted-Lowry definition. • A Lewis acid is an electron pair acceptor. • A Lewis base is an electron pair donor. • Lewis bases are structurally the same as BrØnsted-Lowry bases. Both have an available electron pair—a lone pair or an electron pair in a bond. • A BrØnsted-Lowry base always donates this electron pair to a proton, but a Lewis base donates this electron pair to anything that is electron deficient. 30 • A Lewis acid must be able to accept an electron pair, but there are many ways for this to occur. • All BrØnsted-Lowry acids are also Lewis acids, but the reverse is not necessarily true. o Any species that is electron deficient and capable of accepting an electron pair is also a Lewis acid. • Common examples of Lewis acids (which are not BrØnstedLowry acids) include BF3 and AlCl3. These compounds contain elements in group 3A of the periodic table that can accept an electron pair because they do not have filled valence shells of electrons. 31 • Any reaction in which one species donates an electron pair to another species is a Lewis acid-base reaction. • In a Lewis acid-base reaction, a Lewis base donates an electron pair to a Lewis acid. • Lewis acid-base reactions illustrate a general pattern in organic chemistry. Electron-rich species react with electronpoor species. • In the simplest Lewis acid-base reaction one bond is formed and no bonds are broken. This is illustrated in the reaction of BF3 with H2O. H2O donates an electron pair to BF3 to form a new bond. 32 • A Lewis acid is also called an electrophile. • When a Lewis base reacts with an electrophile other than a proton, the Lewis base is also called a nucleophile. In this example, BF3 is the electrophile and H2O is the nucleophile. electrophile nucleophile 33 • Two other examples are shown below. Note that in each reaction, the electron pair is not removed from the Lewis base. Instead, it is donated to an atom of the Lewis acid and one new covalent bond is formed. 34 • In some Lewis acid-base reactions, one bond is formed and one bond is broken. To draw the products of these reactions, keep in mind the following steps: Always identify the Lewis acid and base first. Draw a curved arrow from the electron pair of the base to the electron-deficient atom of the acid. Count electron pairs and break a bond when needed to keep the correct number of valence electrons. 35 Consider the Lewis acid-base reaction between cyclohexene and H—Cl. The BrØnsted-Lowry acid HCl is also a Lewis acid, and cyclohexene, having a bond, is the Lewis base. 36 • To draw the product of this reaction, the electron pair in the bond of the Lewis base forms a new bond to the proton of the Lewis acid, generating a carbocation. • The H—Cl bond must break, giving its two electrons to Cl, forming Cl¯. • Because two electron pairs are involved, two curved arrows are needed. 37 Which is not a Lewis base? NH3 Lewis base must have an electron pair to donate. Which is not a Lewis acid? Br OH CH B Br Br None, all are Lewis acids b/c they either contain an unfilled valence shell or an acidic proton. 38 39