Decision One:
advertisement
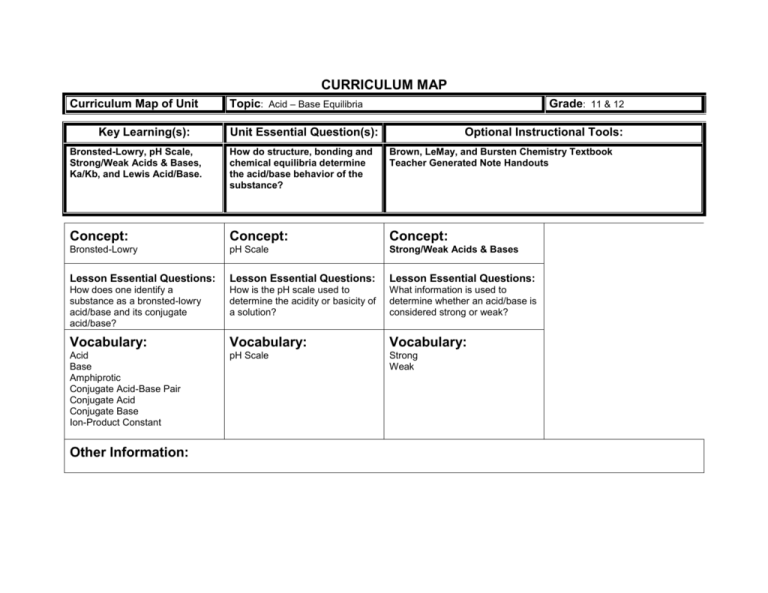
CURRICULUM MAP Curriculum Map of Unit Key Learning(s): Topic: Acid – Base Equilibria Grade: 11 & 12 Unit Essential Question(s): Optional Instructional Tools: Bronsted-Lowry, pH Scale, Strong/Weak Acids & Bases, Ka/Kb, and Lewis Acid/Base. How do structure, bonding and chemical equilibria determine the acid/base behavior of the substance? Brown, LeMay, and Bursten Chemistry Textbook Teacher Generated Note Handouts Concept: Concept: Concept: Bronsted-Lowry pH Scale Strong/Weak Acids & Bases Lesson Essential Questions: Lesson Essential Questions: Lesson Essential Questions: How does one identify a substance as a bronsted-lowry acid/base and its conjugate acid/base? How is the pH scale used to determine the acidity or basicity of a solution? What information is used to determine whether an acid/base is considered strong or weak? Vocabulary: Vocabulary: Vocabulary: Acid Base Amphiprotic Conjugate Acid-Base Pair Conjugate Acid Conjugate Base Ion-Product Constant pH Scale Strong Weak Other Information: CURRICULUM MAP Curriculum Map of Unit Key Learning(s): Topic: Acid – Base Equilibria Unit Essential Question(s): Bronsted-Lowry, pH Scale, Strong/Weak Acids & Bases, Ka/Kb, and Lewis Acid/Base. How do structure, bonding and chemical equilibria determine the acid/base behavior of the substance? Concept: Concept: Ka / Kb Lewis Acid/Base Lesson Essential Questions: Lesson Essential Questions: If you know the Ka or Kb of a weak acid/base, what other information can be obtained? What characteristics determine if a substance is a Lewis acid or base? Vocabulary: Vocabulary: Acid Ionization Constant Polyprotic Acid %Ionization/%Dissociation Base Ionization Constant Binary Acids Ternary Acids Oxoacids Other Information: Grade: 11 & 12 Optional Instructional Tools: Brown, LeMay, and Bursten Chemistry Textbook Teacher Generated Note Handouts











