Chemistry Bonding & Structure Worksheet
advertisement

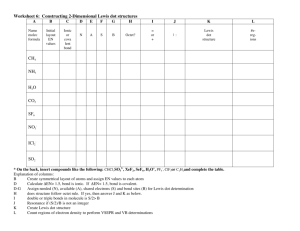
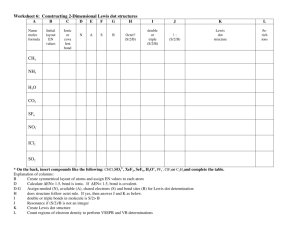
Draw the correct Lewis dot structure for NaCl Draw the correct Lewis dot structure for CH2O & determine the shape Trigonal Planar Describe the structure of metallic bonding. •Positive metallic ions surrounded by electrons. Example of Metallic Bonding Potassium bromide forms a crystal lattice structure. What type of bonding would you expect it have? •Ionic Write the electron configuration for the element potassium 2 2 6 2 6 1 •1s 2s 2p 3s 3p 4s Write the noble gas configuration for the element rubidium 1 •[Kr]5s Write the orbital notation for the element carbon ↑↓ ↑↓ 1s 2s ↑ ↑ 2p . What type of bond exists between chlorine (EN = 3.0) and bromine (EN = 2.8)? Give a reason using electronegativity. •Covalent; difference in electronegativity is small (less than 1.67) Bent Draw the correct Lewis dot structure for SCl2 & determine the shape Which of these elements would make a covalent bond with nitrogen? Al Mg Ba •C C Which element has the following electron configuration: 1s22s22p63s23p64s23d6 •Iron, Fe Write the noble gas configuration for the element chlorine 2 5 •[Ne]3s 3p Write the orbital notation for the element sulfur ↑↓ 1s ↑↓ 3s ↑↓ ↑↓ ↑↓ ↑↓. 2s 2p ↑↓ ↑ ↑ . 3p Which of these elements would make an ionic bond with potassium? Mg N Sr •N & Cl Cl Draw the correct Lewis Dot Structure for CO2 & determine the shape Linear Which of these elements would make an ionic bond with chlorine? Ni F Li •Ni and Li Xe Write the electron configuration for the element fluorine •1s22s22p5 Which element has the following electron configuration: 1s22s22p63s23p64s23d104p3 •Arsenic, As What type of bond exists between nitrogen (EN = 3.0) and calcium(EN = 1.00)? Give a reason using electronegativity. •ionic; difference in electronegativity is big (more than 1.67) Write the electron configuration for the element silicon •1s22s22p63s23p2 Write the electron configuration for the element titanium •1s22s22p63s23p64s23d2 Write the Lewis Dot structure for the following atoms Aluminum Phosphorus Helium