AP Chemistry---Chemical Bonding--
advertisement

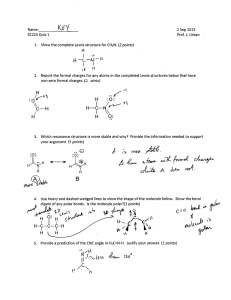
AP Chemistry---Chemical Bonding--- Free Response Exam Questions Please write all responses on the answer sheet. Each question is worth 10 points. 1. Draw the Lewis structure of XeF4. Name the geometry of this molecule. Identify the hydridization of Xe in this molecule. Is this a polar substance? Explain. . 2. Draw the Lewis structures of each of the following. Determine all formal charges and rank each in terms of carbon to oxygen bond length. Indicate resonance structures if appropriate. How many sigma and pi bonds are in CO? CO 3. CO2 CO32- Draw the Lewis structures for the following substances and clearly label all bond angles. Name the VSEPR geometry of each molecule. ClF3 PF5