Galvanic Cell Concept
advertisement

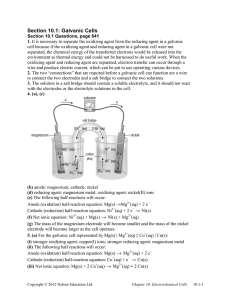
Galvanic Cell Concept • Separating the oxidation and reduction half-reactions will make it so the energy in the electron transfer can be harnessed. • A salt bridge or porous disk is needed to connect the half-reactions so ions can flow and electrons don’t build up on one side of the reaction (one beaker) • **VIDEO? Galvanic Cell Definition • Device which chemical energy is changed to electrical energy. • Oxidation occurs at the ANODE • Reduction occurs at the CATHODE (cat gets fat = cathode gains electrons a.k.a. reduction) – An ox and a red cat (anode/oxidation, reduction/cathode) Electrodes • If there is an element (not ion) in either half-reaction, it is what that particular electrode is made of ***comes up later! • When all reactants/products are in solution (aq) Pt or graphite can be used Galvanic Cell Picture (Parts) Cell Potential/Electromotive Force • (EMF) Represented by E°cell • Unit = volt (V) = 1 joule/coulomb • Measured with a voltmeter (not completely accurate b/c of heat). A potentiometer is used instead where the maximum cell potential can be measured. Standard Reduction Potentials • If we can find the potential for each halfreaction (Table 18.1 pg. 829), we can determine the cell potential (E°cell) • Half-reaction manipulations (DO NOT MANIPULATE VOLTAGE): – One must be reversed (oxidation)…can reverse E so you have -E = -voltage… – Electrons lost must = electrons gained, so multiplication of reaction may be needed (DO NOT MULTIPLY VOLTAGE BY THIS NUMBER!) • EQUATION (MEMORIZE) E°cell = E°(cathode) - E°(anode) Table 18.1 • Better oxidizing agents: easily reduced, LEFT side rxn. = largest, most positive standard reduction potential • Better reducing agents: easily oxidized, RIGHT side = most negative standard reduction potential (aka most positive standard oxidizing potential) • Example: Which is best reducing agent Cu+, F-, H-, H2O, I2, K (find in reactants) • Answer: K (-2.92) > H- (-2.23) > Cu+ (0.16) > I2 (1.20) > H2O (1.23) > F- (2.92) Galvanic Cell Example • Calculate the emf values (E°cell) for the following Mg(s) + 2H+(aq) -> Mg2+(aq) +H2(g) • Answer: E°cell = +2.37V In Galvanic Cells… • When E°cell is positive, the reaction will run spontaneously. (last slide) • If negative, it will run in the opposite direction (will NOT run spontaneously as written). Cell Potential, Electrical Work, and Free Energy • Spontaneous IF: – Positive cell potential – Negative Gibbs Free Energy (we will learn more about Gibbs Free Energy in a later chapter) Line Notation • Not required for AP Exam • A double vertical line separates the anode on left and cathode on right – Represents a salt bridge or porous disk • A single vertical line separates different phases • Ex: anode Cd -> Cd2+ + 2ecathode Hg2+ + 2e- -> Hg Line notation: Cd(s) I Cd2+(aq) II Hg2+(aq) I Hg Galvanic Cell: Complete Description In half-reaction descriptions…FOUR items needed: 1. The cell potential - positive when E°cell = E°(cathode) - E°(anode) and the balanced cell reaction 2. The direction of electron flow, obtained by inspecting the half-reactions and using the direction that gives a positive E°cell. 3. Designation of the anode and cathode. 4. The nature of each electrode and the ions present in each compartment. A chemically inert conductor is required if none of the substances participating in the half-reaction is a conducting solid. Example: Complete Description • Describe a galvanic cell based on the two halfreactions below. Cu2+ + 2e- -> Cu E° = 0.34 V Cr2O72- + 14H+ + 6e- -> 2Cr3+ + 7H2O E° = 1.33V 1. Balanced cell rxn: 3Cu(s) + Cr2O72-(aq) + 14H+(aq) -> 3Cu2+(aq) + 2Cr3+(aq) + 7H2O(l) E°cell = 0.99V (needs to be positive) 2. 1.33 (cathode) -0.34 (anode) means Cu needs to be reversed. Cu will be giving off e- which will travel from Cu (anode) to cathode (platinum electrode). Continued… 3. Anode (copper metal electrode), cathode (platinum electrode) 4. The copper metal electrode (anode) will be in the Cu/Cu2+ compartment while the platinum electrode (cathode) will be in the Cr2O72-/Cr3+ compartment