Chapter 18
advertisement

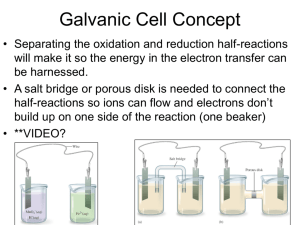
Definition • The study of the interchange of chemical and electrical energy. • Involve oxidation-reduction reactions Half-Reaction Method • Used to balance redox rxns in aqueous solution • One of the rxns is the oxidation rxn • Other is the reduction rxn • The half reactions are balanced separately and then added • Differs in acidic vs. basic solution In Acidic Solution 1. Separate the oxid. and red. parts of the rxn. 2. For each: a. Balance all elements but H and O b. Balance O using H2O c. Balance H using H+ d. Balance charge using electrons 3. If needed, mult. One or both half-rxns. by a whole # to make electrons equal for both 4. Add half-rxns and cancel same on both sides 5. Make sure elements/charges are balanced Acronym for Balancing in Acid • Big White Hogs Eat Bagels and Cream Cheese • • • • • • B = balance all but H & O W = add water for O’s H = Add H E = Add electrons B = Make sure electrons match CC = Combine and cancel Example in Acid • Balance the following equation in acid solution using the half-reaction method. Cu(s) + HNO3(aq) -> Cu2+(aq) + NO(g) • Answer: 3Cu + 2HNO3 + 6H+ -> 3Cu2+ + 2NO + 4H2O Cr2O72-(aq) + NO(g) -> Cr3+(aq) + NO3-(aq) • Answer: Cr2O72-(aq) + 2NO(g) + 6H+(aq) -> 2Cr3+(aq) + 2NO3(aq) + 3H2O(l) In Basic Solution • Follow the steps used in acidic solution THEN… • Add OH- to match H+ on same side. Add to other side as well • Form H2O by adding OH- and H+ • Cancel H2O’s on both sides • Check for element/charge balance Example in Base • Balance the following equation in basic solution: P4 -> PH3 + HPO32• Answer P4 + 2H2O + 4OH- -> 2PH3 + 2HPO32- Galvanic Cell Concept • Separating the oxidation and reduction half-reactions will make it so the energy in the electron transfer can be harnessed. • A salt bridge or porous disk is needed to connect the half-reactions so ions can flow and electrons don’t build up on one side of the reaction (one beaker) • **VIDEO? Galvanic Cell Definition • Device which chemical energy is changed to electrical energy. • Oxidation occurs at the ANODE • Reduction occurs at the CATHODE (cat gets fat = cathode gains electrons a.k.a. reduction) – An ox and a red cat (anode/oxidation, reduction/cathode) Electrodes • If there is an element (not ion) in either half-reaction, it is what that particular electrode is made of ***comes up later! • When all reactants/products are in solution (aq) Pt or graphite can be used Galvanic Cell Picture (Parts) Cell Potential/Electromotive Force • (EMF) Represented by E°cell • Unit = volt (V) = 1 joule/coulomb • Measured with a voltmeter (not completely accurate b/c of heat). A potentiometer is used instead where the maximum cell potential can be measured. Standard Reduction Potentials • If we can find the potential for each halfreaction (Table 18.1 pg. 829), we can determine the cell potential (E°cell) • Half-reaction manipulations (DO NOT MANIPULATE VOLTAGE): – One must be reversed (oxidation)…can reverse E so you have -E = -voltage… – Electrons lost must = electrons gained, so multiplication of reaction may be needed (DO NOT MULTIPLY VOLTAGE BY THIS NUMBER!) • EQUATION (MEMORIZE) E°cell = E°(cathode) - E°(anode) Table 18.1 • Better oxidizing agents: easily reduced, LEFT side rxn. = largest, most positive standard reduction potential • Better reducing agents: easily oxidized, RIGHT side = most negative standard reduction potential (aka most positive standard oxidizing potential) • Example: Which is best reducing agent Cu+, F-, H-, H2O, I2, K (find in products) • Answer: K (-2.92) > H- (-2.23) > Cu+ (0.16) > I2 (1.20) > H2O (1.23) > F- (2.92) Galvanic Cell Example • Calculate the emf values (E°cell) for the following Mg(s) + 2H+(aq) -> Mg2+(aq) +H2(g) • Answer: E°cell = +2.37V In Galvanic Cells… • When E°cell is positive, the reaction will run spontaneously. (last slide) • If negative, it will run in the opposite direction (will NOT run spontaneously as written). Cell Potential, Electrical Work, and Free Energy • Spontaneous IF: – Positive cell potential – Negative Gibbs Free Energy (we will learn more about Gibbs Free Energy in a later chapter) Line Notation • Not required for AP Exam • A double vertical line separates the anode on left and cathode on right – Represents a salt bridge or porous disk • A single vertical line separates different phases • Ex: anode Cd -> Cd2+ + 2ecathode Hg2+ + 2e- -> Hg Line notation: Cd(s) I Cd2+(aq) II Hg2+(aq) I Hg Galvanic Cell: Complete Description In half-reaction descriptions…FOUR items needed: 1. The cell potential - positive when E°cell = E°(cathode) - E°(anode) and the balanced cell reaction 2. The direction of electron flow, obtained by inspecting the half-reactions and using the direction that gives a positive E°cell. 3. Designation of the anode and cathode. 4. The nature of each electrode and the ions present in each compartment. A chemically inert conductor is required if none of the substances participating in the half-reaction is a conducting solid. Example: Complete Description • Describe a galvanic cell based on the two halfreactions below. Cu2+ + 2e- -> Cu E° = 0.34 V Cr2O72- + 14H+ + 6e- -> 2Cr3+ + 7H2O E° = 1.33V 1. Balanced cell rxn: 3Cu(s) + Cr2O72-(aq) + 14H+(aq) -> 3Cu2+(aq) + 2Cr3+(aq) + 7H2O(l) E°cell = 0.99V (needs to be positive) 2. 1.33 (cathode) -0.34 (anode) means Cu needs to be reversed. Cu will be giving off e- which will travel from Cu (anode) to cathode (platinum electrode). Continued… 3. Anode (copper metal electrode), cathode (platinum electrode) 4. The copper metal electrode (anode) will be in the Cu/Cu2+ compartment while the platinum electrode (cathode) will be in the Cr2O72-/Cr3+ compartment Changing Concentration • In chemical reactions… • Standard conditions = 1M for all • When reactants are >1M, it will increase product concentration and will increase the cell potential • When products are >1M, it will decrease the product concentration (oppose forward reaction), decreases cell potential • Mg(s) + 2H+(aq) -> Mg2+(aq) +H2(g) Concentration Cell • A galvanic cell that has the same component on each side but at different concentrations • Causes a cell potential • Voltages are typically small • Ex: a cell has on its left side a 0.20 M Cu2+ solution and a 0.050 M Cu2+ solution on the right side Reaction Quotient • Represented by Q • Q = concentration of productsx [reactants]y • *Solids and liquids are not included… • Multiply concentrations of products/reactants by one another • X and Y represent coefficients of each reactant and product respectively Reaction Quotient Example (13.5) H2(g) + I2(g) <-> 2HI(g) [H2]o = 0.81 M, [I2]o = 0.44 M, [HI]o = 0.58 M Q = __[HI]2_ = [H2]1[I2]1 (0.58)2 (0.81)(0.44) = 0.94 Nernst Equation • At 25°C…to find actual cell potential • Ecell = E°cell - 0.0591 log(Q) n • n is number of moles of electrons transferred • Q is the reaction quotient (see previous slide) • E°cell is calculated using standard reduction potentials (learned earlier this chapter) Nernst Equation Example • Calculate Ecell for a galvanic cell based on the following half-reactions at 25C. (Eq 1) FeO42- + 8H+ + 3e- -> Fe3+ + 4H2O E° = +2.20 V (Eq 2) O2 + 4H+ + 4e- -> 2H2O E° = +1.23 V [FeO42-] = 2.0 X 10-3 M, [Fe3+] = 1.0 X 10-3 M, [O2] = 1.0 X 10-5 M, [H+] = 6.31 X 10-6 M (see pg. 399 in study guide) E = 0.54 V Ion-Selective Electrodes • Cell potential is related to concentrations • Electrodes can be used that are sensitive to specific ions • They measure concentrations of specific ions which can have an effect on the cell potential Batteries • Groups of galvanic cells connected which add together to give the total battery potential • Different types… Lead Storage Battery • Used in cars • Lead = anode • Lead coated with lead dioxide = cathode • Electrodes are dipped in sulfuric acid Dry Cell Batteries • Calculators, electronic games, digital watches, portable audio players, etc. • Acid version, alkaline version, silver cell, mercury cell, etc. Fuel Cells • Reactant constantly supplied to a galvanic cell • Reaction is: 2H2(g) + O2(g) 2H2O(l) Corrosion • Metals corrode because they oxidize easily (spontaneously) – The metals’ reduction potentials are less positive than oxygen gas – When half reactions are combined, there is a positive E° value – Speed of oxidation varies – Some metals form a thin protective oxide coating, preventing further erosion Iron Corrosion • Steel corrosion must be prevented b/c it makes the backbone of most of our buildings, bridges, and cars • Chemical composition of steel is not uniform – Places where iron is easily oxidized/reduced • Ions and electrons migrate via moisture on steel’s surface, causing rust • Moisture acts as a salt bridge between the anode/cathode regions Corrosion Prevention • A coating (paint/metal plating) is applied to protect the metal from oxygen and moisture – Chromium/tin used to plate steel with an oxide coating – Zinc used in galvanizing steel forms an oxidecarbonate coating. Oxygen reacts with the zinc (sacrificial coating) instead of the metal underneath • Alloying: covers metals with a thin layer of stainless steel or other desirable alloy More Corrosion Protection… • Cathodic protection: for buried tanks/pipes attaches an active metal (like Mg) loses electrons, keeping iron from oxidizing – Ships’ hulls work this way: bars of titanium attached to the steel hull keep the hull from being oxidized Electrolysis • Electrolytic cells use electrical energy to produce a chemical change (requires energy) – Electrolysis forces a current through a cell to produce a chemical change for which the cell potential is negative – Causes a nonspontaneous rxn to occur • Charges batteries, produces aluminum metal, plates objects with chrome • Switches the anode/cathode, flows in the opposite direction as the galvanic cell Stoichiometry in Electrochemistry • Can measure how much chemical change occurs with the flow of a given current for a specific amount of time • We add current and time to the chemical equation and can use the mole to mole ratio to do problems • Important Conversion Factor for Ampere (amp): 1 A = coulomb/second • 1 Faraday: 1 mole e- = 96,486 C Stoichiometry Example • How many grams of copper can be reduced by applying a 3.00 A current for 16.2 min to a solution containing Cu2+ ions? • Answer: 0.96 g Cu Plating Order • Plating means depositing neutral metal on the electrode by reducing the metal ions in solution • More positive (higher) E° value will plate out quickest • Ex: pg. 851 Electrolysis Used Commercially… • • • • Production of Aluminum Electrorefining of Metals Metal Plating Electrolysis of Sodium Chloride Production of Aluminum • Aluminum used to not be able to be produced by humans (very expensive) • Hall-Heroult process uses molten cryolite (Na3AlF6) as a solvent for aluminum oxide • Made aluminum easy (cheap) to produce • Production of Al uses 5% of the total electricity used in the U.S. Electrorefining of Metals • Purifies metals like Cu, Fe, Zn, Ag, Au, Pt Metal Plating • Metals that corrode easily can be plated with a thin coating of a metal that is less likely to corrode • Ex: “tin” cans = steel cans with thin tin coating, bumpers in cars are steel coated in chrome • Object needing coating serves as the cathode in ions of the coating metal Electrolysis of Sodium Chloride • • • • How sodium metal is primarily produced NaCl mixed with CaCl2 to lower mp Downs cell used for electrolysis Pg. 856