Balancing Redox Reactions
advertisement

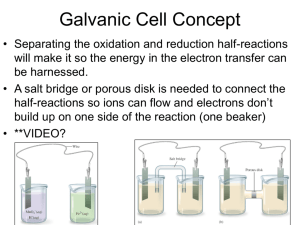
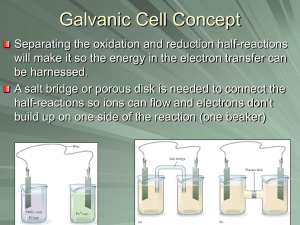
Chapter 18 Electrochemistry The batteries we use to power portable computers and other electronic devices all rely on redox reactions to generate an electric current. Redox reactions are central to the development of small, light, long-lasting power sources. To a large extent, the future development of technology depends on the capabilities of these power sources. In this chapter, we see what is involved in using chemical reactions to generate electricity. Assignment for Chapter 18 18.2; 18.6; 18.17; 18.27 Electrochemistry in Biological Systems Ion transport Muscle contraction Neuron excitation Psychological activities Electrochemistry • Electrochemistry is a branch of chemistry that deals with the use of spontaneous chemical reactions to produce electricity and the use of electricity to bring about spontaneous chemical change. Why electricity and chemical reaction may be connected? Because both involve electrons! The motion or transfer of electrons! Proton transfer: acid-base reactions Electron transfer: redox reactions Half-Reactions • Chemical equations showing the changes involved only in oxidation or reduction. Oxidation half-reaction: 2 Mg(s) Mg (aq) 2e Reduction half-reaction: Fe (aq)+3e Fe(s) 2+ - Example The oxidation of iron(II) to iron(III): Fe (aq) Fe (aq)+e 2+ 3+ - The reduction of copper(II) to copper metal: Cu (aq)+2e Cu(s) 2+ - Exercise: Aluminum metal is oxidized to Al3+ in aqueous solution: Al(s) Al (aq)+3e 3+ - Balancing Redox Reactions MnO4-(aq) + H2C2O4 (aq) Mn2+ (aq) + CO2 (g) ! • The chemical equation of a reduction halfreaction is added to that of an oxidation half-reaction to form the chemical equation for the overall redox reaction. Use H2O and H+ and OH- as “extra” chemicals Balancing Redox Reactions in acidic solutions MnO4-(aq) + H2C2O4 (aq) Mn2+ (aq) + CO2 (g) ! Reduction: MnO4- Mn2+ (aq) Oxidation: H2C2O4 (aq) CO2 (g) Balance all elements except H and O: Reduction: MnO4- Mn2+ (aq) Oxidation: H2C2O4 (aq) 2CO2 (g) Balance O using water and H using H+: Reduction: MnO4- + 8H+ Mn2+ (aq)+4H2O Oxidation: H2C2O4 (aq) 2CO2 (g) + 2H+ Balancing Redox Reactions Balance Charge: Reduction: MnO4- + 8H+ +5e- Mn2+ (aq)+4H2O Oxidation: H2C2O4 (aq) 2CO2 (g) + 2H+ + 2eReduction x 2 + Oxidation x 5 2MnO4- + 16H+ +10e- 2Mn2+ (aq)+8H2O 5H2C2O4 (aq) 10CO2 (g) + 10H+ + 10e2MnO4- (aq)+ 5H2C2O4 (aq) +6H+ (aq) 2Mn2+ (aq)+8H2O(l) + 10CO2 (g) Figure 18.1 A schematic diagram of how to balance a redox equation by balancing the half-reactions separately and then combining them. Example • Write down the balanced net ionic reaction for the reaction: Cu+HNO3Cu2++NO. Cu+H++NO3-Cu2++NO CuCu2+ 2NO3-2NO + 4H2O CuCu2+ 8H++2NO3-2NO + 4H2O CuCu2++2e- 8H++2NO3-+6e-2NO + 4H2O x3 3Cu(s)+2NO3-(aq)+8H+(aq)3Cu2+(aq)+2NO(g)+4H2O(l) Exercise MnO4++H2SO3 HSO4- + Mn2+. Write down the balanced net ionic reaction. Balancing Redox Reactions in basic solutions Br- (aq)+ MnO4-(aq) MnO2(s)+BrO3-(aq) Br- BrO3- MnO4-(aq) MnO2(s) Br- + 3H2OBrO3- MnO4-(aq) MnO2(s) +2H2O Br- + 3H2O+6OH-BrO3-+6H2O MnO4-(aq)+4H2O MnO2(s) +2H2O+4OHBr- + 6OH-BrO3-+3H2O +3eMnO4-(aq)+2H2O MnO2(s) +4OH-+6e2MnO4-(aq)+ Br-(aq) +H2O(l) MnO2(s)+BrO3-(aq)+2OH-(aq) Electrochemical Cell • A device in which an electric current is either produced by a spontaneous chemical reaction or is used to bring about a nonspontaneous reaction. A galvanic cell is an electrochemical cell in which a spontaneous chemical reaction is used to generate an electric current. Figure 18.2 In an electrochemical cell, a reaction takes place in two separate negative regions. Oxidation occurs at one electrode, and the electrons released travel through the external circuit to the other electrode, where they cause reduction. The site of oxidation is called the anode, and the site of reduction is called the cathode. positive Any two objects that have different (first) ionization energies may function as a cell. - 1.234 + - 0.02 + Figure 18.3(a) When a bar of zinc is placed in a beaker of copper(II) sulfate solution, copper is deposited on the zinc and the blue copper (II) ions are gradually replaced by colorless zinc ions. (b) The residue in the beaker is copper metal. No more copper ions can be seen in solution. Zn(s)+Cu (aq) Zn (aq)+Cu(s) 2+ 2+ Figure 18.4 The reaction shown in Fig. 18.3 takes place all over the surface of the zinc as electrons are transferred to the Cu2 ions in solution. Cu 2+ (aq)+2e- Cu(s) Zn(s) Zn (aq)+2e 2+ - Figure 18.5 The Daniell cell consists of copper and zinc electrodes dipping into solutions of copper(II) sulfate and zinc sulfate, respectively. The two solutions make contact through the porous pot, which allows ions to pass through to complete the electrical circuit. Zn(s)|Zn 2+ Cu 2+ |Cu(s) Zn(s) Zn 2+ (aq)+2e- Cu (aq)+2e Cu(s) 2+ - Electrodes and Cell Diagram Zn(s)|Zn 2+ Cu 2+ |Cu(s) + H (aq)|H 2 (g)|Pt(s) 3+ 2+ Fe (aq),Fe (aq) |Pt(s) 2+ 2+ Zn(s)|Zn (aq)|Cu (aq) |Cu(s) Figure 18.6 This cell is typical of galvanic cells used in the laboratory. The two electrodes are connected by an external circuit and a salt bridge. The latter completes the electrical circuit within the cell. Pt(s)|Fe3+ (aq),Fe2+ (aq) ||Cu 2+ (aq) |Cu(s) Figure 18.7 The cell potential is measured with an electronic voltmeter, a device that draws negligible current so that the composition of the cell does not change during the measurement. The display shows a positive value when the terminal of the meter is connected to the cathode of the galvanic cell. 2+ 2+ Zn(s)|Zn (aq)||Cu (aq) |Cu(s) Cell Potential E = 1.1 V The cell potential • An indication of the electron-pulling and – pushing power of the cell reactions; cell reactions at equilibrium generate zero potential. Figure 18.8 Electrons produced by oxidation leave a galvanic cell at the anode (), travel through the external circuit, and reenter the cell at the cathode (), where they cause reduction. The circuit is completed inside the cell by migration of ions through the salt bridge. A salt bridge is unnecessary when the two electrodes share a common electrolyte. positive negative Figure 18.9 This schematic picture of a galvanic cell indicates the identities of the anode and cathode, displays the oxidation and reduction half-reactions, and shows the direction of electron flow. Describing a galvanic cell and identifying the cell reaction Hg 2+ (aq)+2e- 2Hg(l) 2Hg(l)+2Cl- (aq) Hg 2Cl2 (s)+2e- Hg (aq)+2Cl (aq) Hg 2Cl2 (s) 2+ - Hg(l)|Hg 2Cl 2 (s)|HCl(aq)||Hg (NO ) (aq) |Hg(l) 2 3 2 (KCl gel) Exercise: Describing a galvanic cell and identifying the cell reaction (Assume platinum electrode is used) H 2 (g) 2H + (aq)+2e- Co3+ (aq)+e- Co2+ (aq) H2 (g)+2Co (aq) 2H (aq)+ 2Co (aq) 3+ + + 3+ 2+ 2+ Pt(s)|H 2 (g)||H (aq)||Co (aq),Co (aq) |Pt(s) Figure 18.12 The cell potential can be thought of as being the difference of the two reduction potentials produced by the two electrodes. The cell potential is positive if the cathode has a higher potential than the anode. Cell potential and electrode potential standard cell potential: E 0 E 0 (cathode) E 0 (anode) Fe(s)|Fe 2+ (aq)||Ag + (aq) | Ag(s): E 0 1.24 V Hydrogen standard potential 2H +2e H 2 (g): E 0 V + - 0 Zn(s)|Zn (aq)||H (aq) | H 2 (g)|Pt(s): E 0.76 V 2+ + 0 E 0 E 0 (H + / H 2 ) E 0 (Zn 2+ / Zn) 0.76 V E 0 (Zn 2+ / Zn) 0.76 V Figure 18.13 The variation of standard potentials in the main groups of the periodic table. Note that the most negative values occur in the s block and the most positive values occur close to fluorine. Example: deducing the standard potential of an electrode The standard potential of a Zn2+/Zn electrode is -0.76 V and the standard potential of the cell Zn(s)|Zn2+(aq)||Cu2+(aq)|Cu(s) is 1.10 V. What is the standard potential of Cu2+/Cu electrode? E 0 (Cu 2+ /Cu) E 0 (Zn 2+ /Zn) 1.10 V E (Cu / Cu ) 1.10 V E (Zn / Zn) 1.10 V - 0.76 V=+0.34 V 0 2+ 0 2+ Exercise: deducing the standard potential of an electrode The standard potential of a Fe2+/Fe electrode is -0.44 V and the standard potential of the cell Fe(s)|Fe2+(aq)||Pb2+(aq)|Pb(s) is 0.31 V. What is the standard potential of Pb2+/Pb electrode? E 0 (Pb2+ /Pb) E 0 (Fe2+ /Fe) 0.31 V E ( Pb / Pb) 0.31 V+E ( Fe / Fe) 0.31 V 0.44 V =-0.13 V 0 2+ 0 2+ Figure 18.14 The significance of standard potentials. Only couples with negative standard potentials (and hence lying below hydrogen) can reduce hydrogen ions to hydrogen gas. The reducing power increases as the standard potential becomes more negative. E 0 (Cu 2+ / Cu)=+0.34 V E (Cu /Cu) E (H /H) 0.34 V 0 2+ 0 + Cu (aq)+H 2 (g) Cu(s)+H (aq) 2+ + E 0 (Zn2+ / Zn)=-0.76 V E (Zn /Zn) E (H /H) 0.76 V 0 2+ 0 + Zn(s)+H (aq) Zn (aq)+H 2 (g) + 2+ Figure 18.15 Although aluminum has a negative standard potential, signifying that it can be oxidized by hydrogen ions (as in the hydrochloric acid, left), nitric acid (right) stops reacting with it as soon as an impenetrable layer of aluminum oxide has formed on its surface. This resistance to further reaction is termed passivation of the metal. E 0 (Al3+ /Al)=-1.66 V E (Al /Al) E (H /H) 1.66 V 0 3+ 0 + Al(s)+H (aq) Al (aq)+H 2 (g) + 3+ There are no images in this section of the chapter. Figure 18.16 The relation between the standard potential of a reaction (reactants, purple; products, yellow) and the equilibrium constant. Investigating Matter 18.1 (a) A glass electrode in a protective plastic sleeve (left) is used to measure pH. It is sometimes used in conjunction with a calomel electrode (right) in pH meters such as this one. Investigating Matter 18.1 (b) This durable portable pH meter can be used for quick measurements of pH in the field. Its accuracy is not as high as that of a laboratory pH meter. Figure 18.17 A commercial dry cell consists of a graphite cathode in a zinc container; the latter acts as the anode. The container is filled with a moist paste of NH4Cl, MnO2, finely divided carbon, and an inert filler such as starch. In the cell reaction, manganese(IV) is reduced to manganese(III) and zinc metal is oxidized to Zn2 ions. Figure 18.18 One cell of a leadacid battery like those used in automobiles. A lead-acid battery is an example of a secondary cell. It needs to be charged before it can produce a current. The electrolyte is dilute sulfuric acid. Figure 18.19 A rechargeable nickel-cadmium (nicad) cell. The electrodes are assembled in a jelly roll arrangement and separated by a layer of paper soaked in moist sodium or potassium hydroxide. Figure 18.20 The electric eel (Electrophorus electricus) lives in the Amazon. The average potential difference it produces along its length (1 m) is about 700 V. Case Study 18 (a) One of the three alkali fuel cells used on the space shuttle. Although only one cell is needed to provide lifesupport, electricity, and drinking water, shuttle flight rules require that all three be functioning. Case Study 18 (b) High-pressure hydrogen tanks run across the top of this bus provided by Ballard Power Systems for testing hydrogen-oxygen fuel cells in Chicago. The bus is pollution free and can go 250 miles before needing to be refueled. Figure 18.21 Iron nails stored in oxygen-free water (left) do not rust because the oxidizing power of water itself is weak. When oxygen is present (as a result of air dissolving in the water, right), oxidation is thermodynamically spontaneous and rust soon forms. Figure 18.22 The mechanism of rust formation. (a) Oxidation of the iron occurs at a point out of contact with the oxygen of the air, and the surface of the metal acts as an anode in a tiny galvanic cell. (b) Further oxidation of Fe2 to Fe3 results in the deposition of rust on the surface. Figure 18.23 Metal girders are galvanized by immersion in a bath of molten zinc. Fig. 18.24 In the cathodic protection of a buried pipeline, or other large metal construction, the artifact is connected to a number of buried blocks of metal, such as magnesium or zinc. The sacrificial anodes (the magnesium block in this illustration) supply electrons to the pipeline (the cathode of the cell), thereby preserving it from oxidation. Figure 18.25 A schematic picture of the electrolytic cell used in the Dow process for magnesium. The electrolyte is molten magnesium chloride. As the current generated by the external source passes through the cell, magnesium metal is produced at the cathode and chlorine gas is produced at the anode. Figure 18.26 In this schematic picture of an electrolysis experiment, the electrons emerge from a galvanic cell at its anode () and enter the electrolytic cell at its cathode (), where they bring about reduction. Electrons are drawn out of the electrolytic cell through its anode () and into the galvanic cell at its cathode (). If the cell reaction in the galvanic cell is more strongly spontaneous than the reaction in the electrolytic cell is nonspontaneous, then the overall process is spontaneous. This experiment is an example of one reaction driving another to which it is coupled. Figure 18.27 Michael Faraday (1791–1867) giving a public lecture on chemistry at the Royal Institution in London. Figure 18.28 A schematic picture showing the electrolytic process for refining copper. The anode is impure copper. It undergoes oxidation, and the Cu2 ions so produced migrate to the cathode, where they are reduced to pure copper metal. A similar arrangement is used for electroplating objects. Figure 18.29 A schematic representation of the stoichiometric relations that are used to calculate the amount of product formed by electrolysis or the amount of time current must flow to produce a given product. Figure 18.30 In the Downs process, molten sodium chloride is electrolyzed with a graphite anode (at which the Cl ions are oxidized to chlorine) and a steel cathode (at which the Na ions are reduced to sodium). The sodium and chlorine are kept apart by the hoods surrounding the electrodes. Calcium chloride is present to lower the melting point of sodium chloride to a more economical value. Figure 18.31 Chromium plating lends decorative flair as well as protection to the steel of this motorcycle. Large quantities of electricity are needed to plate chromium because six electrons are required to produce each atom of chromium. Nobel Prize in Chemistry 2007 Assignment for Chapter 18 18.2; 18.6; 18.17; 18.27