Chem 214 CH 13 Fundamentals of Electrochemistry Lecture 2
advertisement

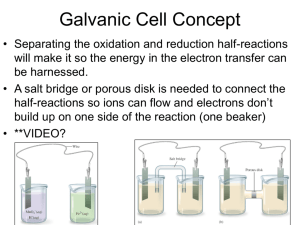
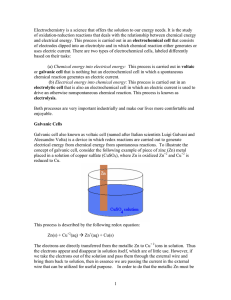
Fundamentals of Electrochemistry Lecture 2 of x, Chapter 13 1 Activity 1 Understanding Galvanic Cells 2 Galvanic Cells A galvanic cell uses a spontaneous chemical reaction to generate electricity. What are the key components? Anode? Cathode? Salt bridge? 3 Drawing a Galvanic Cell Can you sketch out the Danielle Cell in its entirety? 4 Analyze This Cell Anode? Cathode? To force electrons to go through potentiometer, the two electrodes must not be contact. Look in the diagram. How are the electrodes separated? Half-reactions at each electrode? How is this cell different from Danielle cell? 5 Analyze This Cell Does this galvanic cell work? Meaning do electrons flow from anode, potentiometer, and cathode? Why or why not? What happens at the surface of Cd electrode when Cd oxidizes? 6 Line Diagram A line diagram is a notation for describing a galvanic cell. | phase boundary (e.g., solid to liquid) || salt bridge (e.g., two different liquids) What is a line diagram for the Danielle cell? Note anode is written at extreme left-side of line and cathode at extreme right-side. 7 Problem 13-8 Write a line notation and two reduction half-reactions for each cell pictured above. 8 Problem 13-9 Draw a picture of the following cell and write reduction half-reactions for each electrode: 9 Activity 2 The Human Salt Bridge 10 Human Salt Bridge A salt bridge is an ionic medium with a semipermeable barrier on each end. Example: U-tube filled with agar and KCl, filter paper soaked in saltwater, and you! Human body is just a bag of salt housed in a semipermeable membrane (i.e., skin) Question: Can we measure a voltage from a Danielle cell using filter paper, two fingers, and students holding hands. 11 Activity 3 How Do You Make a Battery? 12 Making a Battery Anode? Cathode? Salt bridge? Half-reactions? Materials: Pennies, nickels, paper, salt water, voltmeter 13 14