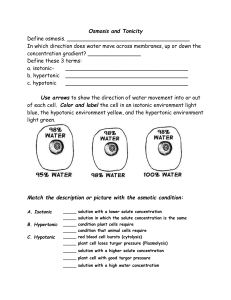
Osmosis lab 2 --
advertisement

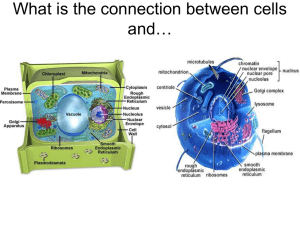
What is the connection between cells and… Desalination plant –Middle East Converts salt water into fresh water Snowboarding parka shell and snow pants Dialysis machine What variables affect the passage of molecules through cell membranes? Draw a cross section of a “typical” cell membrane. Objective • Determine which solution is Isotonic, Hypotonic, Hypertonic ……but you can only test 2 of the 3 solutions Materials – – – – – Distilled water (100% H20) 3 mystery solutions Quadruple beam balances Potatoes cells IKI solution (Lugol’s solution) Some chemistry terms used • Solutions can be solids dissolved in liquids. They could also be gases dissolved in liquids (such as carbonated water). • There can also be gases in other gases and liquids in liquids. If you mix things up and they stay at an even distribution, it is a solution. • A simple solution is basically two substances that are going to be combined. One of them is called the solute. A solute is the substance to be dissolved (sugar). The other is a solvent. The solvent is the one doing the dissolving (water). As a rule of thumb, there is usually more solvent than solute. Homework • Speculate on the possible effects of molecular concentration on movement through cell membranes. Using a model cell- design an experiment or experiments to test your ideas. Construct a pre-lab flowchart for your experimental design. Day of Lab • After your pre-lab flowchart has been stamped off for completion, decide as a group the different experiments that you will run to test your hypotheses. • Professional points will be lost for groups who do not clean up their stations • Record all of your results. Graph your quantitative data and be ready to present your findings to the class in 30 minutes. • Did the class results reveal a consistent pattern? If so, what was it? • Which of the proposed hypotheses were supported by the experimental findings? Explain • Homework complete question 11 After Lab • Draw a cross section of your model cell membrane. Label your diagram. • Include this in your FDQ lab write up solution, hypertonic, n a mixture containing a concentration of solute in excess of the concentration of the same solute in another mixture to which it is compared. When the two solutions are placed on opposite sides of a permeable membrane (either artificial or natural, as with cell membranes), the hypertonic solution attracts the solvent from the hypotonic solution, equalizing the concentration of the solute in both. See also solution, hypotonic; solution, isotonic; and osmosis. solution, hypotonic, n a mixture containing a concentration of solute that is lower than the concentration of the same solute in another mixture to which it is compared. When two such solutions are separated by a permeable membrane, the solvent of the hypotonic solution flows through the membrane to the hypertonic solution, equalizing the concentration of the solute in both. See also solution, hypertonic; solution, isotonic; and osmosis. solution, isotonic, n a mixture containing the same concentration of solute as another mixture to which it is compared. When separated by a permeable membrane, osmosis does not occur. See also solution, hypertonic; solution, hypotonic; and osmosis.