Experiment 11:
advertisement

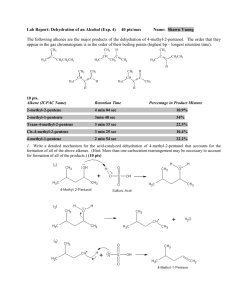
Experiment 10: ACID-CATALYZED DEHYDRATION OF AN ALCOHOL WITH REARRANGEMENT Objectives To perform a dehydration of 2-methylcyclohexanol to form isomeric alkenes under E1 conditions. To purify the product using simple distillation. To analyze the product using GC analysis in order to identify and quantify products. To characterize the reactant and products using IR spectroscopy. Before coming to lab… Please review: Simple distillation E1 elimination reactions GC Analysis CHEMICAL EQUATION • Three different ISOMERIC alkenes can be produced. • Because this reaction is performed near equilibrium conditions, the relative amount of each product reflects its stability. OH CH3 CH3 CH3 + + CH2 H2SO4 heat more substituted = lower energy = more stable = more predominate! RATE LIMITING STEP + H OSO3H .. C C H OH .. Fast Reaction C C H O+ H H Rate limiting step C C HSO4- C C + H C C + H C C H O+ H H + HSO4- + H2O + H2SO4 Fast Reaction • The rate of elimination of water depends on the stability of the carbocation formed. • Formation of the carbocation is the most energetically unfavorable, and therefore the slowest, step in dehydration reactions. E1 MECHANISM 2. …which forms a new O-H bond, where oxygen bears a positive charge (oxonium ion). Water is eliminated-forms 2o carbocation. 1. The hydroxyl oxygen attacks and removes a proton from sulfuric acid… H OH CH3 H H 3. Products may form from the 2o carbocation, but it is more likely that the 2o C+ will rearrange to a 3o C+. O H O SO3H H H a 2o CH3 CH3 H H + H2O + HSO4 secondary carbocation + HSO4- H b H H H CH3 d c) Hydride Shift H 2O H a H Carbocation rearrangement H H H e d 3o C H b a) -H3O+ 1-methyl-1cyclohexene CH3 b) -H3O+ d) -H3O+ CH3 3-methyl-1cyclohexene 1-methyl-1cyclohexene 4. At the carbocation stage, water will remove a proton from the carbon ADJACENT to the carbocation. The electrons form the pi bond of the alkene. H H e H2O e) -H3O+ CH2 CH3 methylenecyclohexane THEORETICAL YIELD The only reactant is 2-methylcyclohexanol. The H2SO4 is simply a catalyst, since it is regenerated in the end. Theoretical yield is calculated assuming that the major product formed is one that results from the most stable carbocation intermediate. Theoretical yield (g) = # g reactant 1 mol of reactant #g Amount you started with Molecular weight of reactant 1 mol product 1 mol reactant Stoichiometric ratio #g 1 mol product Molecular weight of product Always end up in units of grams of product!!! OVERVIEW Set up and perform simple distillation to collect products. Obtain final product mass and calculate percent yield. Prepare and submit GC sample for analysis. Pick up GC results and record standard retention times. Identify components in sample chromatogram by comparing to standard chromatogram. Quantify alkenes by calculating adjusted area percent. Characterize reactant and products using provided IR spectra. EXPERIMENTAL PROCEDURE: (Simple distillation) Clamp flask to ring stand here! Blue Keck clips here! Clamp flask to ring stand here! water out • Place 2-methylcyclohexanol, sulfuric acid and boiling chips in 50 mL round bottom flask. • Clamp flask to ring stand. water in • Weigh 10 mL flask. Clamp to other ring stand. 50 mL Heating Mantle 10 mL • Attach clear hoses to condenser. Run water in at the bottom, out at the top! iron ring to voltage regulator • Build rest of distillation apparatus, using blue Keck clips to secure top and bottom joints around condenser. EXPERIMENTAL PROCEDURE: (Simple distillation) • Begin water flow, and apply heat (VR@30) to boil solution. • Record temperature when distillate begins to collect in 10 mL flask (Ti). water out water in • Collect ~ 5 mL distillate. Keck clips! • Record temperature right before you drop the heating mantle (Tf). Heating Mantle • Allow the solution to cool. iron ring to voltage regulator • Reweigh 10 mL flask to obtain actual product yield. • Prepare GC sample and submit! Don’t forget!!! Table 10.1: Experimental Results must calculate the amount of product that can be Theoretical Yield (g) formed based on the amount of 2methylcyclohexanol used! This mass will be obtained by weighing the 10 mL Actual Yield (g) round bottom flask before and after the distillation. The difference in the mass is the actual product yield. % yield Actual yield (g) X 100 Theoretical yield (g) physical state and color of distillate. Product Appearance Table 10.2: GC Analysis Results Compound GC Retention time (min) Standard methanol Sample Area Percent Adjusted Area Percent Never calculate adjusted area % based on the solvent! 2-methylcyclohexanol No need to calculate adjusted area % on the reactant, either! 1-methyl-1-cyclohexene Area % THIS alkene Sum area% all alkenes X 100 3-methyl-1-cyclohexene Area % THIS alkene Sum area% all alkenes X 100 methylenecyclohexane Area % THIS alkene Sum area% all alkenes X 100 Infrared Spectroscopy (IR) Q: What is it? Vibrational energy of bonds Certain types of polar bonds absorb IR radiation and vibrate (excited state) Q: Why is it useful? Certain functional groups absorb at characteristic frequencies. By looking at what frequencies are absorbed, we can identify the presence or absence of certain types of bonds! Infrared Spectroscopy (IR) Q: How does it work? This molecule is represented with a potential energy diagram. Each horizontal line represents a vibrational state of a C=O bond. If we add IR light energy at the correct wavelength, we get excitation to the next vibronic energy level. Infrared Spectroscopy (IR) Q: What is an IR spectrum? % transmittance of IR radiation Frequency of vibration (in wavenumbers) EXPERIMENTAL PROCEDURE: IR Analysis THINGS TO CONSIDER… OH CH3 CH3 •What kinds of bonds do I have? • Ex. C-O, C=C, CH3, etc. • If they appeared in the IR spectrum, where would they be? • Use a correlation table to determine the approximate frequency for that type of bond. • Now, look at the spectrum. Are they there? EXPERIMENTAL PROCEDURE: IR Analysis Full IR Absorption Correlation Table in Appendix J Base values for Absorptions of Bonds (cm-1) OH ~3400 C-O ~1100 C-H (sp2) ~3100-3000 C-H (sp3) ~3000-2850 C=C ~1630 Table 10.3: IR Spectral Analysis Results IR spectra are on page 87 in lab manual! Functional Group 2-methylcyclohexanol 1-methyl-1cyclohexene 3-methyl-1cyclohexene Methylenecyclohexane Frequency (cm-1) Frequency (cm-1) Frequency (cm-1) Frequency (cm-1) 3200-3500 N/A N/A N/A 1000-1200 N/A N/A N/A Base Values (cm-1) OH stretch C-O stretch 2850-3000 sp3 CH stretch 3000-3100 N/A 1600-1680 N/A sp2 CH stretch C=C stretch Infrared Spectroscopy (IR) (How to answer the questions…) Your goal is to explain clearly how you were able to use IR spectroscopy to DIFFERENTIATE between reactant and product. Always discuss the appearance of certain types of absorptions, or the disappearance of others, which indicate that functional groups have changed. Always answer like this: (fill in the blanks) In the IR spectrum of the product, the appearance of the _____ (type of bond) absorption at _____ (actual frequency) indicates the conversion of the reactant to the product. The typical frequency for this type of absorption is _____ (base value frequency). SAFETY CONCERNS • The alcohol and resulting alkenes are extremely flammable. Be very cautious when applying heat. • Concentrated sulfuric acid is VERY CORROSIVE and will burn skin on contact. Please use gloves and goggles at all times when in laboratory. WASTE MANAGEMENT Place all liquid waste in the container labeled “LIQUID WASTE”. Be careful when disposing of acidic waste remaining in 50 mL round bottom flask! It is extremely corrosive. Use a small amount of water to rinse it into the waste container before cleaning it thoroughly using directions on next slide… CLEANING After disposing of the liquid waste, clean the 50 mL round bottom flask with soap, water, brush, and a final rinse with wash acetone. All other ground glass joint ware can simply be rinsed with wash acetone into a waste container. Be sure all ground glass joint ware is completely dry before returning to plastic container in fume hood. Be sure all other glassware used is completely dry before returning to lab drawer. IN LAB QUESTIONS (The following questions should be answered in laboratory notebook.) Predict the products and draw a complete mechanism for their formation from acid-catalyzed dehydration of 2-methyl-2-pentanol. Be sure to show all steps and intermediates. Circle the major product. HO + MW: 102.18 g/mol H O SO3H ? IN LAB QUESTIONS (The following questions should be answered in laboratory notebook.) Calculate the theoretical yield for the reaction above based on 4.0 g of the starting alcohol and a catalytic amount of sulfuric acid. The molecular weight of the alcohol is given, but the molecular weight of the product must be determined based on the structure. Be sure to include units.