Periodic Table Basics & Terminology
advertisement

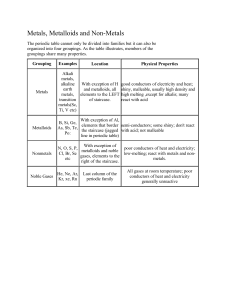
Mr. Chapman Science 9 Norquay School : Basics & Terminology The periodic table is composed of rows and columns of elements. Some important definitions: Element – an element cannot be reduced to a simpler substance by normal means. An atom is a certain type of element based on its number of protons. Examples: Compound – a compound is a pure substance composed of two or more different elements. Examples: Mr. Chapman Science 9 Norquay School Periodic Table Basic Information A row on the periodic table is called a period. A column on the periodic table is called a group. A group on the period table is often referred to as a chemical family. All of the elements in a chemical family typically react in a very similar way, because each element in the family has the same number of valence electrons. There are 4 important chemical families for you to remember: 1. Alkali metals (group 1) – the alkali metals are very soft, and are very reactive. They react violently with water to form an alkaline solution. 2. Alkaline-earth metals (group 2) – these metals are not as soft as group 1 metals, and not quite as reactive. However, they still react with water to form new compounds. 3. Halogens (group 17) – the halogens are extremely reactive, because they only need one electron to fill their outer shell. They are gases at room temperature. 4. Noble gases (group 18) – the noble gases are called noble because they do not react with other elements. This is because they have a full outer shell of electrons. Mr. Chapman Science 9 Norquay School The transition metals are in the middle block of the periodic table. The periodic table is also divided into metals and non-metals by a staircase, which can be found on the right side of the periodic table. Everything on the left of the staircase (except hydrogen) is a metal, and everyone on the right of the staircase is a non-metal. Metalloids are elements that are right along the staircase, in between metals and non-metals. Mr. Chapman Science 9 Norquay School Please complete the following questions for the periodic table: 1. Identify each of the following elements as either a metal or a non-metal: Carbon – Oxygen – Gold – Magnesium – Phosphorus – 2. What is another name for a chemical group? 3. What family do each of the following elements belong to? Sodium – Beryllium – Bromine – Xenon – 4. Would you expect potassium or calcium to react more intensely with water? Explain your answer.