Section 3.3 – Phases Changes
advertisement

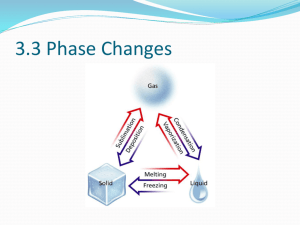
Section 3.3 – Phases Changes Key Concepts (you should be able to answer these questions) • What are six common phase changes? • What happens to a substance’s temperature and a system’s energy during a phase change? • How does the arrangement of water molecules change during melting and freezing? • How are evaporation and boiling different? Important Vocabulary (leave room in your notes for definitions!) • • • • • • • • • • • Phase change Endothermic Exothermic Heat of fusion Vaporization Heat of vaporization Evaporation Vapor pressure Condensation Sublimation Deposition Phase Changes • When more than one state of matter is present in a system, scientists say there are two different phases of matter – Example: an iceberg floating in the ocean • A phase change is a reversible physical change that occurs when a substance goes from one form of matter to another • Melting, freezing, vaporization, condensation, sublimation and deposition are six common phase changes Phase Changes - continued • All phase changes share certain characteristics related to energy and temperature • The temperature of a substance does not change during a phase change – This means one way we can recognize a phase change is by measuring the temperature of a substance as it is heated or cooled Phase Change Diagram for Water What happens during phase changes? • Energy gets transferred between a substance and its surroundings • Energy is either absorbed or released during a phase change • In an endothermic reaction, the substance absorbs energy from its surroundings • In an exothermic reaction, the substance releases energy into its surroundings Endothermic/Exothermic Reactions • An example of an endothermic change is ice melting – Where does the energy for melting come from? • 1 gram of ice absorbs 334 Joules [J] of energy as it melts • This amount of energy is the heat of fusion, or how much energy is needed to be absorbed for 1 gram of the substance to melt, for water. • Note that the heat of fusion varies from substance to substance. • What do you think happens when ice freezes? • Application: Preventing crop freezing Melting • The arrangement of molecules in water becomes less orderly as water melts and more orderly as water freezes. • Melting is change of a solid into a liquid. Since energy is absorbed, this change is endothermic. • For ice, molecules gain energy, which means they vibrate more quickly until they gain enough energy that they are able to overcome the forces of attraction from the other molecules and move from their fixed positions. Freezing • Freezing is the change of a liquid into a solid. Since energy is released, the change is exothermic. • As the average kinetic energy decreases because it is being released into the system, the molecules of a substance move more slowly until the forces of attraction start to have an effect and pull them into an orderly arrangement. • Often you think of freezing happening at cold temperatures, but things freeze when they turn from a liquid into a solid, so they can freeze at really high temperatures. Vaporization • Vaporization is the change of a liquid into a gas. Since a substance absorbs energy, it is endothermic. • 1 gram of water needs 2261 Joules of energy in order to vaporize. – This is the heat of vaporization, or how much energy is required for one gram of a substance to change from a liquid to a gas, for water. • The heat of vaporization changes from substance to substance. Two types of vaporization • The two types of vaporization are evaporation and boiling. • Evaporation occurs when some of the molecules near the surface of a liquid are moving fast enough to overcome the forces of attraction from other molecules. – A larger surface area means something can evaporate faster. • Examples of evaporation? Evaporation • In a closed container, water evaporates into water vapor and collects above the liquid. • Vapor pressure is the pressure caused by the collisions of the vapor with the walls of the container. • If the temperature of the vapor increases, the vapor pressure increases as well. Boiling • Boiling occurs when the vapor pressure of a system is equal to the atmospheric pressure. • The temperature at which this happens is the boiling point. • As you increase the temperature of something you are trying to boil, you increase the vapor pressure. • When you boil a pot of water, the molecules move faster and faster until the molecules below the surface of the water have enough kinetic energy to overcome the forces of attraction from neighboring molecules. • Since vapor is less dense than the liquid, it rises to the surface and the bubbles burst, releasing the vapor into the air. Boiling continued • Note that the boiling point of something depends on atmospheric pressure. At standard pressure, the boiling point of water is 100°C. • The higher your altitude, the lower atmospheric pressure is, which means water will boil at a lower temperature. Condensation • Condensation is the change from a gas into a liquid. Since this transfers heat into a system from the substance, it is exothermic. • Examples? Vaporization / Condensation Application • Refrigeration! Sublimation and Deposition • Sublimation is the phase change from a solid to a gas/vapor without becoming a liquid first. Since this change absorbs energy, it is endothermic. • Deposition is the phase change from a gas/vapor into a solid without becoming a liquid first. Since this change releases energy into a system, it is exothermic. • Examples? Exit Ticket • • • • What are the 6 types of phase changes? Which ones are exothermic? Which ones are endothermic? Provide an example of each.