Chapter 3.3 phase changes
advertisement
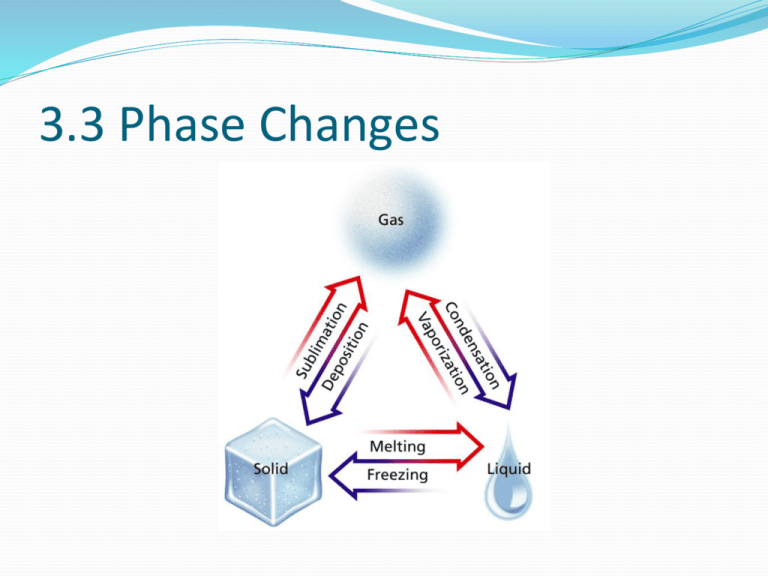
3.3 Phase Changes What are we learning? Define phase change Explain how temperature can be used to recognize a phase change Explain what happens to the motion, arrangement and average kinetic energy of water molecules during phase changes Describe each of the 6 phase changes Identify phase changes as endothermic or exothermic Characteristics of a Phase Change If 2 states of the same substance are present at the same time, we describe each different state as a phase. Ex: an iceberg floating in the ocean- solid phase and liquid phase A phase change is the reversible physical change that occurs when a substance changes from one state of matter to another Melting, freezing, vaporization, condensation, sublimation and deposition are the 6 common phase changes Temperature and Phase Changes One way to recognize phase changes- measuring the temperature as it is heated or cooled The temperature of a substance does not change during a phase change. Ex Naphthalene Naphthalene is used in mothballs. This graph is of data collected when it is heated. The temperature rises as it warms up, until it hits about 80C. (the melting point) It will remain at 80C until it has all melted . Energy and Phase Changes During a phase change, energy is transferred between a substance and its surroundings. Energy is either absorbed or released during a phase change. Ex: Melting- endothermic change During an Endothermic change, the system absorbs energy from its surroundings. The amount of energy absorbed varies Ex: one gram of ice absorbs 334 joules of energy as it melts. (this is the heat of fusion of water) Fusion is another term for melting Energy and Phase Changes When water freezes, it releases the same amount of energy (334J) to its surroundings. Freezing is an exothermic change In exothermic change the system releases energy to its surroundings. Melting and Freezing The arrangement of molecules in water becomes less orderly as water melts and more orderly as water freezes. (remember the BB’s) Attractions between water molecules in ice keep the molecules in fixed positions As ice warms up the molecules vibrate more quickly When ice gets near the melting point (OC), some molecules gain enough energy to over come the attraction with other molecules and move from their fixed positions. (melting is complete when all the molecules can move). Freezing When water is placed in a freezer, energy flows from the water to the air in the freezer, and the water cools down. The kinetic energy decreases and the molecules move more slowly As the molecules slow down, forces of attraction have a greater effect When all the molecules have been drawn into an orderly arrangement, freezing is complete Vaporization and Condensation Vaporization is the phase change where a substance changes from a liquid to a gas it’s an endothermic process which means, that a substance must absorb energy in order to change from a liquid to a gas One gram of water gains 2261 joules of energy- this is the heat of vaporization of water There are 2 vaporization process, boiling and evaporation Evaporation Evaporation occurs at the surface of a liquid and at temperatures lower than the boiling point. Evaporation is the process that changes a substance from a liquid to a gas at temp. below the boiling point. When water evaporates, some molecules near the surface are moving fast enough to escape and become water vapor. The greater the surface area, the faster it evaporates Vapor pressure is the pressure caused by the collisions of water vapor and the walls of a closed container Boiling As you heat up a pot of water, the temperature and vapor pressure of water increases The water boils when the vapor pressure becomes equal to the atmospheric pressure (the temp. at which this happens is the boiling point) As the temp. increases during boiling, the molecules move faster When the temp gets near 100C, some molecules below the surface have enough kinetic energy to overcome the attraction with other molecules Bubbles rise to the surface, burst and release water vapor into the air The boiling point depends on atmospheric pressure (higher altitude = lower BP) Condensation Condensation is the phase change where a substance changes from a gas/vapor to a liquid. (morning dew!) Is an exothermic process (energy releasing) Sublimation and deposition Sublimation the phase change where a substance goes from a solid to a gas/vapor without changing to a liquid first. When a gas /vapor changes directly to a solid without going through the liquid phase it’s called deposition. (exothermic) Causes frost to form on windows