Ch5 Sample Problem #43
advertisement
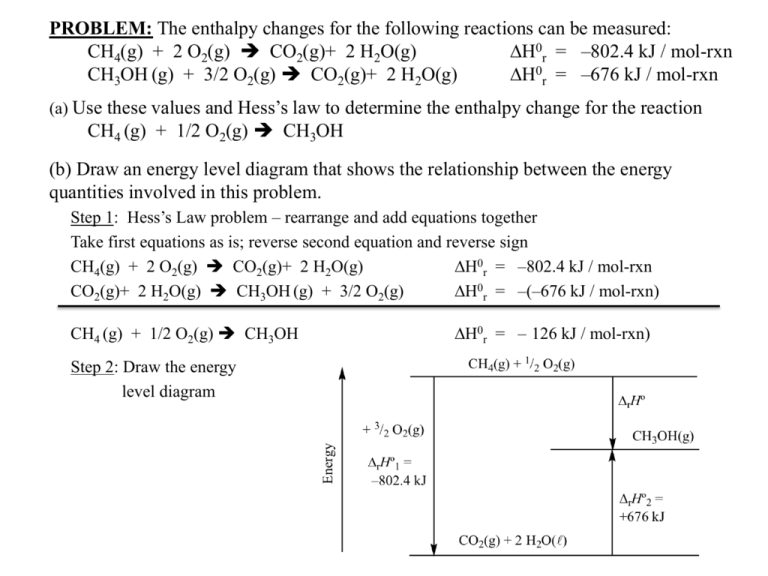
PROBLEM: The enthalpy changes for the following reactions can be measured: CH4(g) + 2 O2(g) CO2(g)+ 2 H2O(g) DH0r = –802.4 kJ / mol-rxn CH3OH (g) + 3/2 O2(g) CO2(g)+ 2 H2O(g) DH0r = –676 kJ / mol-rxn (a) Use these values and Hess’s law to determine the enthalpy change for the reaction CH4 (g) + 1/2 O2(g) CH3OH (b) Draw an energy level diagram that shows the relationship between the energy quantities involved in this problem. Step 1: Hess’s Law problem – rearrange and add equations together Take first equations as is; reverse second equation and reverse sign CH4(g) + 2 O2(g) CO2(g)+ 2 H2O(g) DH0r = –802.4 kJ / mol-rxn CO2(g)+ 2 H2O(g) CH3OH (g) + 3/2 O2(g) DH0r = –(–676 kJ / mol-rxn) CH4 (g) + 1/2 O2(g) CH3OH Step 2: Draw the energy level diagram DH0r = – 126 kJ / mol-rxn)









![Homework V solution [ ][ ] [ ]](http://s2.studylib.net/store/data/013607381_1-076372d3d646781c8f0a3a6699d59ec5-300x300.png)