Halogens TMD
advertisement

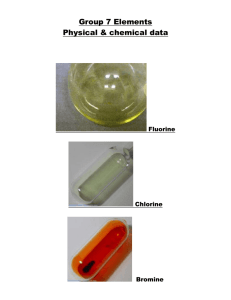
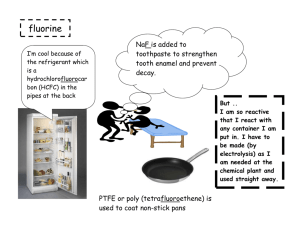
Halogens Objectives Be able to... • Recall the main properties and trends of the Halogens • Explain trends using knowledge of intermolecular bonding and redox • Write half equations for displacement reactions and electrolysis Specification... Where this comes from... GROUP PROPERTIES GENERAL • non-metals • exist as separate diatomic molecules… eg Cl2 • all have the electronic configuration ... ns2 np5 TRENDS • • • • • • • appearance boiling point electronic configuration electronegativity atomic size ionic size oxidising power Physical properties of halogens 5 of 43 © Boardworks Ltd 2009 Trends in boiling point Halogen molecules increase in size down the group. This leads to greater van der Waals forces between molecules, increasing the energy needed to separate the molecules and therefore higher melting and boiling points. van der Waals forces fluorine atomic radius = 42 × 10-12 m boiling point = -118 °C 6 of 43 iodine atomic radius = 115 × 10-12 m boiling point = 184 °C © Boardworks Ltd 2009 Trends in electronegativity Electronegativity of the halogens decreases down the group due to an increase in atomic radius. Increased nuclear charge has no significant effect because there are more electron shells and more shielding. Iodine atoms therefore attract electron density in a covalent bond less strongly than fluorine. fluorine atomic radius = 42 × 10-12 m electronegativity = 4.0 7 of 43 iodine atomic radius = 115 × 10-12 m electronegativity = 2.5 © Boardworks Ltd 2009 Astatine The name astatine comes from the Greek word for unstable. Astatine exists in nature in only very tiny amounts. It is estimated that only 30 grams of astatine exist on Earth at any one time. This is because it is radioactive, and its most stable isotope (210At) has a half-life of only 8 hours. It was first made artificially in 1940, by bombarding 209Bi with a-radiation. What do you predict for these properties of astatine? colour state at room temperature electronegativity. 8 of 43 © Boardworks Ltd 2009 Appearance Halogen Symbol \ Fluorine F Chlorine Cl Bromine Br Iodine I State Colour Colour of vapour Appearance Halogen Symbol State Colour Colour of vapour gas Pale yellow Yellow gas Pale green Green liquid Orange / brown Orange solid Grey-black crystals Purple \ Fluorine Chlorine Bromine Iodine F Cl Br I What would you predict about the appearance of astatine? Physical Properties Fluorine (F2) Chlorine (Cl2) Bromine (Br2) Iodine (I2) What happens to the physical properties as you go down Group VII? Trends in Physical Properties Fluorine (F2) Chlorine (Cl2) Bromine (Br2) Iodine (I2) • Melting points increase • Boiling points increase • All more soluble in organic solvents than water Make sure you can use intermolecular forces to EXPLAIN each of these trends Reactivity of the Group 7 elements Fluorine (F2) Chlorine (Cl2) Bromine (Br2) Iodine (I2) • Decreasing reactivity (This is due to it getting less easy for the atoms to form negative ions by gaining electrons) Make sure you can EXPLAIN this trend in terms of atomic size, shielding and nuclear attraction Displacement Reactions Which of the following reactions will take place? Chlorine Bromine Iodine + Bromine + potassium bromide + potassium chloride potassium chloride + potassium iodide What has to be true for a displacement reaction to take place? Electron structure and reactivity 15 of 43 © Boardworks Ltd 2009 Halogen displacement reactions 16 of 43 © Boardworks Ltd 2009 Halogen displacement reactions 17 of 43 © Boardworks Ltd 2009 Halogen displacement reactions Halogen displacement reactions are redox reactions. Cl2 + 2KBr 2KCl + Br2 To look at the transfer of electrons in this reaction, the following two half equations can be written: Cl2 + 2e- 2Cl- 2Br- Br2 + 2e- What has been oxidized and what has been reduced? Chlorine has gained electrons, so it is reduced to Cl- ions. Bromide ions have lost electrons, so they have been oxidized to bromine. 18 of 43 © Boardworks Ltd 2009 In displacement reactions between halogens and halides, the halogen acts as an oxidizing agent. This means that the halogen: oxidizes the halide ion to the halogen gains electrons is reduced to form the halide ion. What is the order of oxidizing ability of the halogens? increasing oxidizing ability Oxidizing ability of halogens fluorine chlorine bromine iodine 19 of 43 © Boardworks Ltd 2009 Oxidizing ability of halogens 20 of 43 © Boardworks Ltd 2009 Displacement Reactions Writing an ionic equation for a displacement reaction: Br2 + 2I 2Br + I2 • Which species is oxidised in this reaction? • Which species is the oxidising agent? • Which species is the reductant? Displacement Reactions Chlorine can oxidise both bromide and iodide ions: Cl2 + 2BrCl2 + 2I 2Cl- + Br2 2Cl + I2 However, bromine can only oxidise iodide ions: Br2 + 2Cl Br2 + 2I- no reaction 2Br- + I2 So, chlorine is a stronger oxidising agent than bromine. Oxidising ability of the halogens Fluorine (F2) Chlorine (Cl2) Bromine (Br2) Iodine (I2) Decreasing oxidising ability Decreasing reactivity Reducing ability of the halides (F ) Fluoride Chloride (Cl ) Bromide (Br ) Iodide (I ) Increasing reducing ability GROUP TRENDS ELECTRONEGATIVITY Electronegativity F Cl Br I 4.0 3.5 2.8 2.5 DECREASES down Group • the increasing nuclear charge due to the greater number of protons should attract electrons more, but there is an ... an increasing number of shells; more shielding and less pull on electrons an increasing atomic radius attraction drops off as distance increases GROUP TRENDS OXIDISING POWER • halogens are oxidising agents • they need one electron to complete their octet • the oxidising power gets weaker down the group GROUP TRENDS OXIDISING POWER • halogens are oxidising agents • they need one electron to complete their octet • the oxidising power gets weaker down the group • the trend can be explained by considering the nucleus’s attraction for the incoming electron which is affected by the... • increasing nuclear charge which should attract electrons more but this is offset by • INCREASED SHIELDING • INCREASING ATOMIC RADIUS GROUP TRENDS OXIDISING POWER • halogens are oxidising agents • they need one electron to complete their octet • the oxidising power gets weaker down the group • the trend can be explained by considering the nucleus’s attraction for the incoming electron which is affected by the... • increasing nuclear charge which should attract electrons more but this is offset by • INCREASED SHIELDING • INCREASING ATOMIC RADIUS This is demonstrated by reacting the halogens with other halide ions. Reactions with Silver Nitrate Halide Colour of precipitate Formula of precipitate Solubility in ammonia Fluoride no ppt. no ppt. soluble Chloride Bromide Iodide white cream yellow AgCl AgBr AgI soluble partially soluble insoluble How is AgNO3 used to test for halide ions? Which silver halide precipitate is formed most quickly? Can you suggest a use for the silver halides?