Experiment 21

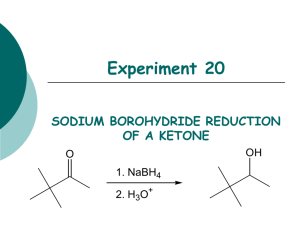
Experiment 20
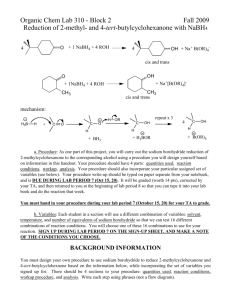
SODIUM BOROHYDRIDE REDUCTION
OF A KETONE
O OH
1. NaBH
4
2. H
3
O
+
Objectives:
To synthesize a secondary alcohol from a ketone using a sodium borohydride reduction.
To purify the product using fractional distillation.
To analyze the purity of the product using
GC.
To characterize the reactants and products using NMR and IR spectroscopy.
Before coming to lab…
Review these techniques:
Fractional distillation
Vacuum filtration
GC Analysis
REDUCTION USING NaBH
NaBH
4
4
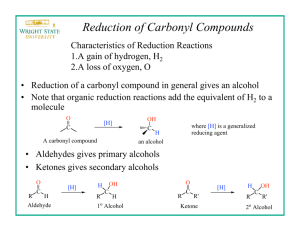
(sodium borohydride) is a versatile and useful reducing agent in organic chemistry.
A reducing agent causes a reaction (a reduction) in which the product has more bonds from carbon to hydrogen (or fewer bond to oxygen)
4 R
O
C R' + 1 NaB H
4 then H
2
O
4 R
O H
C R'
H
MECHANISM
H
3
C
CH
3
O
C C CH
3
CH
3
H
(from
NaB H
4
)
NaBH
4 transfers a hydride ion to the carbonyl carbon.
NaB H
4
H
3
C
CH
3
O
C C
CH
3
H
H
CH
3
H
O
H
(from
6M HCl)
The oxygen anion eventually removes a proton from water.
H
3
C
CH
3
O H
C C CH
3
CH
3
H
TODAY’S REACTION
O
4 (CH
3
)
3
CCCH
3
+ NaB H
4 then H
2
O
Pinacolone
O H
4 (CH
3
)
3
CCCH
3
H
Pinacolone is reduced using sodium borohydride.
Note that ketone.
1 mole of NaBH
4 will reduce
4 moles of
EXPERIMENTAL PROCEDURE
(Synthesis)
Add 3,3-dimethyl-2-butanone and methanol to 100 mL beaker.
Place in ice water bath.
SLOWLY add NaBH with glass rod.
4 while stirring
Continue to react at 0 o C for 5min, then 10 min more at RT.
Add 6M HCl drop wise.
Suction filter to remove solid from liquid filtrate.
Transfer liquid filtrate to 50 mL round bottom flask.
100 mL
50 mL
EXPERIMENTAL PROCEDURE
(Purification and GC Analysis)
Set up fractional distillation apparatus.
Apply heat and collect distillate below 70 o C in a small flask.
Switch to 25 mL round bottom flask and collect distillate between 70-85 o C.
Cool 25 mL flask, reweigh.
Prepare GC sample.
heating mantle to voltage regulator iron ring water out
50mL
Keck clips water in
25mL
PRODUCT!
Table 20.1
Theoretical yield (g)
Actual yield (g)
Percent yield
Distillation Range ( o C)
Product Appearance
Calculate based on limiting reactant
(25mL flask + product)-(empty 25mLflask)
(Actual yield/Theoretical yield) X 100
Give Ti-Tf of distillate collected in 25 mL round bottom flask
Give physical state and color of product
Atom Economy (%)
Table 20.2
Calculate based 3,3-dimethyl-2-butanone and sodium borohydride ONLY!
Experimental
Atom Economy (%)
“E product
”
Cost per synthesis ($)
Cost per gram ($/g) o Review Experiment 13 for calculations!
WASTE MANAGEMENT
o
Place the solid boric acid waste from the filtration into the container labeled “ SOLID WASTE ” located in the waste hood. o
Place all liquid waste into the container labeled
“ LIQUID WASTE ”.
SAFETY CONCERNS
3,3-dimethyl-2-butanone, 3,3dimethyl-2-butanol, methanol and sodium borohydride are all FLAMMABLE materials.
Methanol and sodium borohydride are
TOXIC in large concentrations.
Hydrochloric acid is CORROSIVE .